方案详情文
智能文字提取功能测试中
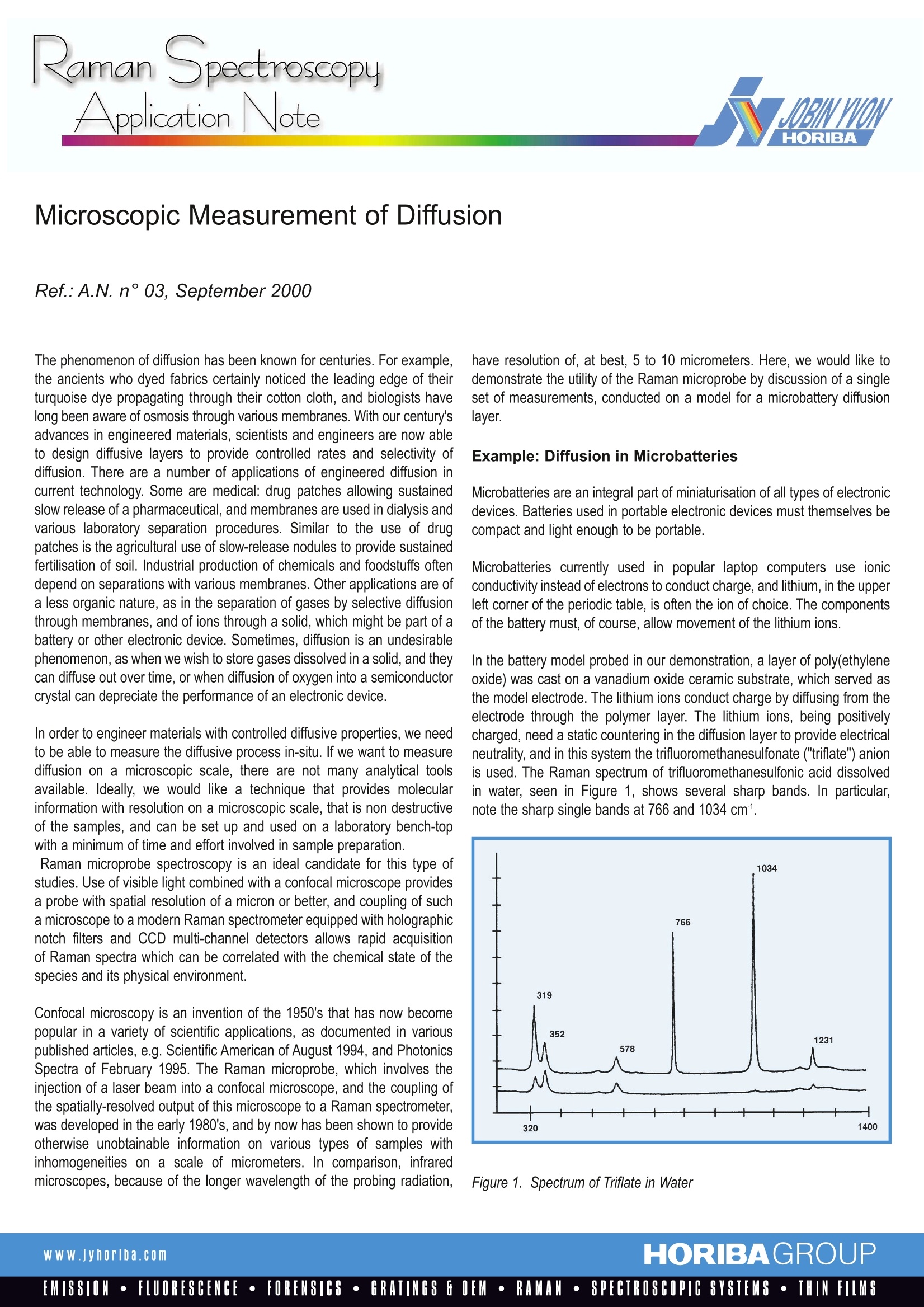
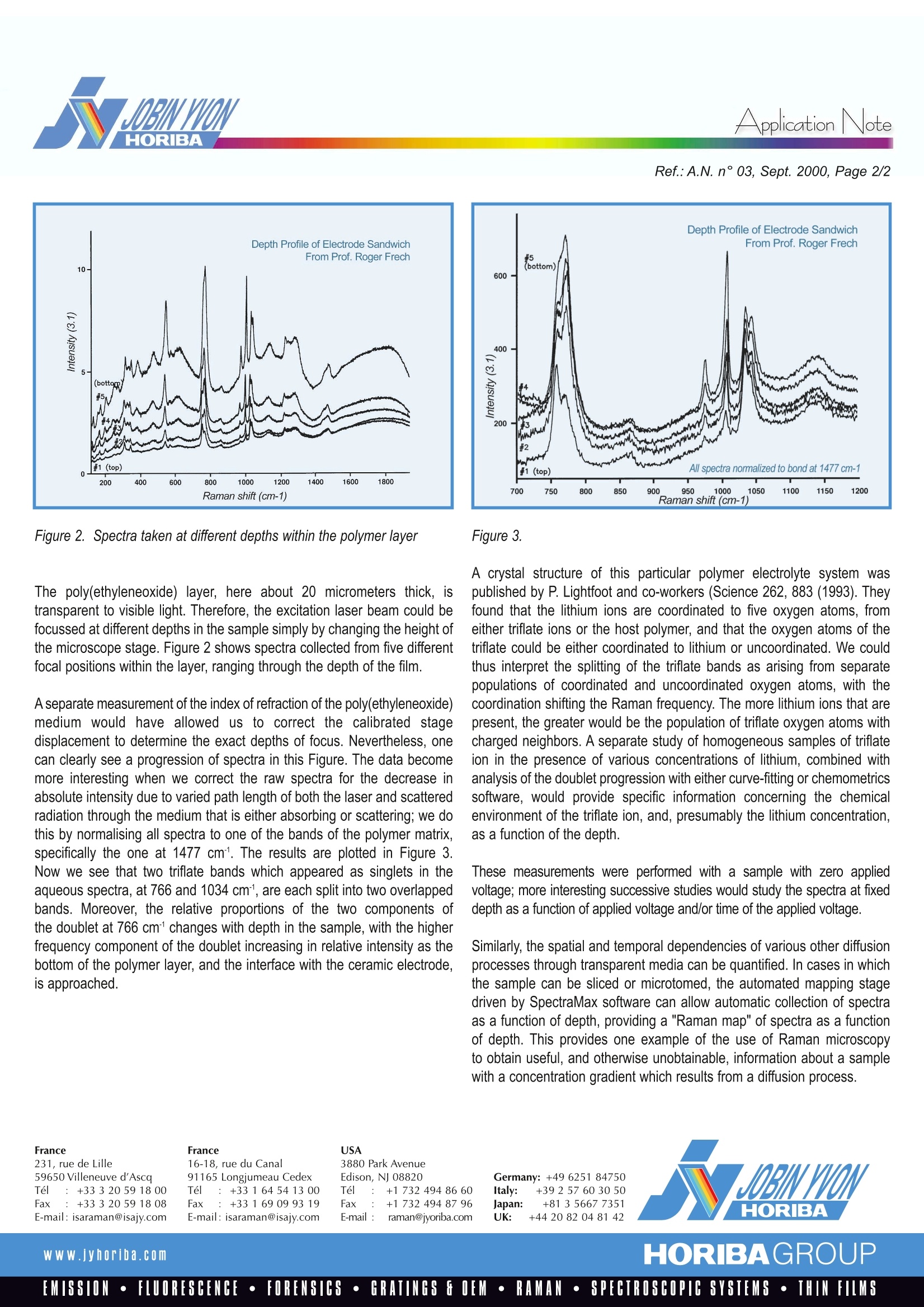
RamanSpectroscopyApplication Note Application NoteRef.:A.N. n°03, Sept. 2000, Page 2/2 Microscopic Measurement of Diffusion The phenomenon of diffusion has been known for centuries. For example,the ancients who dyed fabrics certainly noticed the leading edge of theirturquoise dye propagating through their cotton cloth, and biologists havelong been aware of osmosis through various membranes. With our century’sadvances in engineered materials, scientists and engineers are now ableto design diffusive layers to provide controlled rates and selectivity ofdiffusion. There are a number of applications of engineered diffusion incurrent technology. Some are medical: drug patches allowing sustainedslow release of a pharmaceutical, and membranes are used in dialysis andvarious laboratory separation procedures. Similar to the use of drugpatches is the agricultural use of slow-release nodules to provide sustainedfertilisation of soil. Industrial production of chemicals and foodstuffs oftendepend on separations with various membranes. Other applications are ofa less organic nature, as in the separation of gases by selective diffusionthrough membranes, and of ions through a solid, which might be part of abattery or other electronic device. Sometimes, diffusion is an undesirablephenomenon, as when we wish to store gases dissolved in a solid, and theycan diffuse out over time, or when diffusion of oxygen into a semiconductorcrystal can depreciate the performance of an electronic device. In order to engineer materials with controlled diffusive properties, we needto be able to measure the diffusive process in-situ. If we want to measurediffusion on a microscopic scale, there are not many analytical toolsavailable. Ideally,we would like a technique that provides molecularinformation with resolution on a microscopic scale, that is non destructiveof the samples, and can be set up and used on a laboratory bench-topwith a minimum of time and effort involved in sample preparation. Raman microprobe spectroscopy is an ideal candidate for this type ofstudies. Use of visible light combined with a confocal microscope providesa probe with spatial resolution of a micron or better, and coupling of sucha microscope to a modern Raman spectrometer equipped with holographicnotch filters and CCD multi-channel detectors allows rapid acquisitionof Raman spectra which can be correlated with the chemical state of thespecies and its physical environment. Confocal microscopy is an invention of the 1950's that has now becomepopular in a variety of scientific applications, as documented in variouspublished articles, e.g. Scientific American of August 1994, and PhotonicsSpectra of February 1995.The Raman microprobe, which involves theinjection of a laser beam into a confocal microscope, and the coupling ofthe spatially-resolved output of this microscope to a Raman spectrometer,was developed in the early 1980's, and by now has been shown to provideotherwise unobtainable information on various types of samples withinhomogeneities on a scale of micrometers. In comparison, infraredmicroscopes, because of the longer wavelength of the probing radiation, have resolution of, at best, 5 to 10 micrometers. Here, we would like todemonstrate the utility of the Raman microprobe by discussion of a singleset of measurements, conducted on a model for a microbattery diffusionlayer. Example: Diffusion in Microbatteries Microbatteries are an integral part of miniaturisation of all types of electronicdevices. Batteries used in portable electronic devices must themselves becompact and light enough to be portable. Microbatteries currently used in popular laptop computers use ionicconductivity instead of electrons to conduct charge, and lithium, in the upperleft corner of the periodic table, is often the ion of choice. The componentsof the battery must, of course, allow movement of the lithium ions. In the battery model probed in our demonstration, a layer of poly(ethyleneoxide) was cast on a vanadium oxide ceramic substrate, which served asthe model electrode. The lithium ions conduct charge by diffusing from theelectrode through the polymer layer. The lithium ions, being positivelycharged, need a static countering in the diffusion layer to provide electricalneutrality, and in this system the trifluoromethanesulfonate ("triflate") anionis used. The Raman spectrum of trifluoromethanesulfonic acid dissolvedin water, seen in Figure 1, shows several sharp bands. In particular,note the sharp single bands at 766 and 1034 cm. Figure 3. A crystal structure of this particular polymer electrolyte system waspublished by P. Lightfoot and co-workers (Science 262,883 (1993). Theyfound that the lithium ions are coordinated to five oxygen atoms, fromeither triflate ions or the host polymer, and that the oxygen atoms of thetriflate could be either coordinated to lithium or uncoordinated. We couldthus interpret the splitting of the triflate bands as arising from separatepopulations of coordinated and uncoordinated oxygen atoms, with thecoordination shifting the Raman frequency. The more lithium ions that arepresent, the greater would be the population of triflate oxygen atoms withcharged neighbors. A separate study of homogeneous samples of triflateion in the presence of various concentrations of lithium, combined withanalysis of the doublet progression with either curve-fitting or chemometricssoftware, would provide specific information concerning the chemicalenvironment of the triflate ion, and, presumably the lithium concentration,as a function of the depth. These measurements were performed with a sample with zero appliedvoltage; more interesting successive studies would study the spectra at fixeddepth as a function of applied voltage and/or time of the applied voltage. Similarly, the spatial and temporal dependencies of various other diffusionprocesses through transparent media can be quantified. In cases in whichthe sample can be sliced or microtomed, the automated mapping stagedriven by SpectraMax software can allow automatic collection of spectraas a function of depth, providing a "Raman map" of spectra as a functionof depth. This provides one example of the use of Raman microscopyto obtain useful, and otherwise unobtainable, information about a samplewith a concentration gradient which results from a diffusion process. France231, rue de Lille59650 Villeneuve d'AscaTel :+33320 591800Fax: +33 3 20 59 18 08E-mail: isaraman@isajy.com France16-18, rue du Canal91165 Longjumeau CedexTel :+33164541300Fax: +33169099319E-mail: isaraman@isajy.com 3880 Park AvenueEdison, NJ 08820Tel :+1 7324948660Fax: +1 732494 87 96E-mail :raman@jyoriba.com Germany: +49 6251 84750Italy: +39 257 603050Japan: +81 3 5667 7351UK: +442082048142 HORIBAGROUPwww.jyhoriba.comEMISSION·FLUORESCENCE·FORENSICS·GRATINGS FOEM·RAMAN·SPECTROSCOPIC SYSTEMS· THIN FILMS
关闭-
1/2
-
2/2
产品配置单
HORIBA(中国)为您提供《Microscopic Measurement of Diffusion》,该方案主要用于其他中null检测,参考标准《暂无》,《Microscopic Measurement of Diffusion》用到的仪器有HORIBA XploRA INV多功能拉曼及成像光谱仪、HORIBA XploRA Nano原子力-拉曼联用系统、HORIBA XploRA PLUS超快速拉曼成像光谱仪。
我要纠错
相关方案





 咨询
咨询