方案详情文
智能文字提取功能测试中
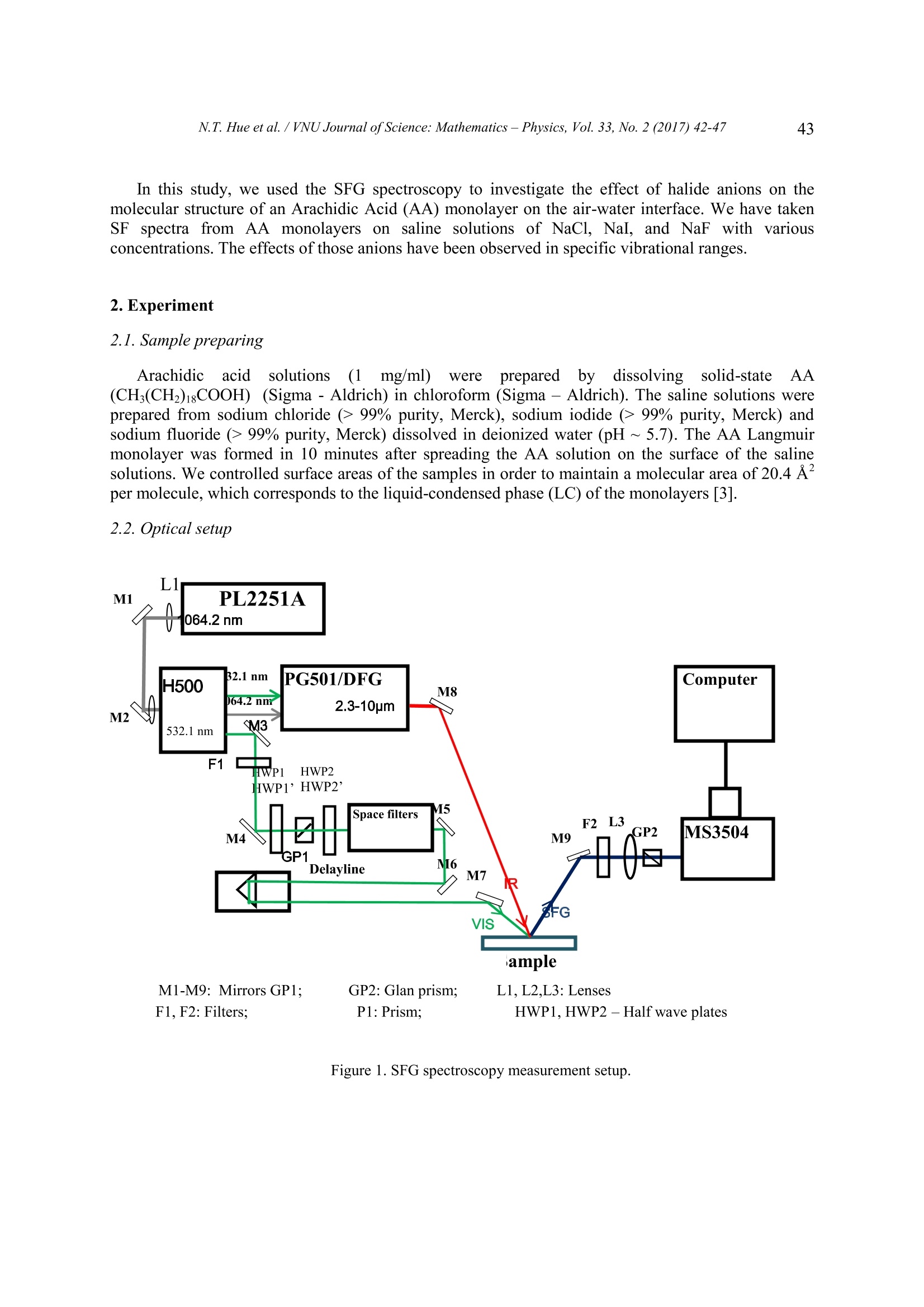
VNU Journal of Science: Mathematics-Physics, Vol. 33, No. 2(2017)42-47 42 Study of Langmuir Monolayers of Arachidic Acidon Saline Solutions Using Sum-frequency GenerationVibrational Spectroscopy Nguyen Thi Hue2, Vu Thi Thanh Tam , Nguyen Anh Tuan 'Faculty ofPhysics, VNU University ofScience, 334 Nguyen Trai, Hanoi, VietnamHung Vuong University, Phu Tho, Vietnam Received 13 March 2017 Revised 28 April 2017; Accepted 25 May 2017 Abstract: In this report, we studied the effect of halide salt concentration on Arachidic Acid (AA)Langmuir monolayers formed on salt solutions, such as NaCl, NaI, NaF by using a Sum-Frequency Generation Vibrational Spectroscopy. In the SFG spectra, we observed the peakintensities of CH3FR and CH3ss vibrational modes decreased when increasing the salt concentration.This observation indicates that the structure of the AA monolayer was disordered by theinteraction of the dissolved ions with the carboxyl head groups of the monolayer. Keywords: Langmuir monolayer, interfacial structure, Sum-frequency vibrational spectroscopy. 1. Introduction Langmuir monolayer is a single monomolecular layer formed on the air/liquid interface. Structuresand properties of Langmuir monolayers are often affected by external conditions such as temperature,dissolved ion concentration, and pH. The fatty acid molecules have both hydrophilic and hydrophobicparts. The head group which contains hydrophilic compounds is attr:1...acted to water. The tail group ofthe hydrocarbon chain is a hydrophobic part, which is out of the water and forms a single layer ofmolecules on the water surface [1]. Langmuir monolayers have attracted studies because theirstructural resembles biological membranes. The interaction in the interface comes from hydrogen-bonding between the head groups and the water molecules. In addition, the presence of dissolved ionsaffects molecular orientation and structure of the interfacial layers. Sum-frequency generation vibrational spectroscopy (SFG-VS) is a second-order nonlinear opticalprocess. Within the dipole approximation, SFG is forbidden in centrosymmetric media. Thus, SFG hasan intrinsic surface/interfacial selectivity. Recently, thanks to the development of ultrafast lasersources, SFG-VS has become a powerful tool to study dynamic phenomena at surfaces andinterfaces [2]. ( Corresponding author. T el.: 84-919148855. ) ( Email: tuanphysics@vnu.edu.vn ) ( https://doi.org/10.25073/2588-1124/vnumap.4202 ) In this study, we used the SFG spectroscopy to investigate the effect of halide anions on themolecular structure of an Arachidic Acid (AA) monolayer on the air-water interface. We have takenSF spectra from AA monolayers on saline solutions of NaCl, NaI, and NaF with variousconcentrations. The effects of those anions have been observed in specific vibrational ranges. 2. Experiment 2.1. Sample preparing Arachidic acidsolutions(1mg/ml)wereeprepared by dissolving solid-state AA(CH,(CH2)18COOH) (Sigma -Aldrich) in chloroform (Sigma -Aldrich). The saline solutions wereprepared from sodium chloride (>99% purity,Merck), sodium iodide (> 99% purity, Merck) andsodium fluoride (> 99% purity, Merck) dissolved in deionized water (pH~5.7). The AA Langmuirmonolayer was formed in 10 minutes after spreading the AA solution on the surface of the salinesolutions. We controlled surface areas of the samples in order to maintain a molecular area of20.4 Apermolecule, which corresponds to the liquid-condensed phase (LC) of the monolayers [3]. 2.2.Optical setup Li M2 M1-M9: Mirrors GP1: GP2: Glan prism; L1, L2,L3: LensesF1,F2: Filters; P1: Prism; HWP1,HWP2 -Half wave plates Figure 1. SFG spectroscopy measurement setup. The optical setup of our SFG spectrometer is shown in figure 1. In this setup, we used a mode-locked Nd:YAG pico-second laser (EKSPLA -PL2251A) as a pump source with wavelength of1064.2 nm, energy of 50 mJ/pulse, pulse width of 30 ps, and repetition rate of 50 Hz. The fundamentalbeam of 1064.2 nm was directed into a second-harmonic unit (H500). The second-harmonic at 532.1nm and the fundamental beam at 1064.2 nm were used to pump an OPG/OPA/DFG system (EKSPLA-PG501). In the DFG block, the 1064.2 nm laser pulses are mixed with idler waves from theOPG/OPA block in a AgGaS2 crystal to obtain tunable waves covering a mid-infrared range from 2.3-10 um. Mirrors M7 and M8 guide the visible (532.1 nm) and the IR beams into the sample at incidentangles of 60° and 55°, respectively. Once the two incident waves satisfied the phase-matchingcondition, SF signal was generated at the reflection angle of 59.7°±0.35°. The SF light was selectedby a monochromator (MS3504) and then detected by photomultiplier tubes. We used a delay line inthe visible path to ensure a temporal overlap at the sample. All of the spectra were taken in SSPpolarization combination. 3. Results and discussion We have taken SFG spectra from AA monolayer/saline solutions of NaI, NaCl, and NaF withvarious saline concentrations. Those spectra, shown in Fig. 2, Fig. 3, and Fig. 4,are dominated by thesymmetric stretching mode of CH3ss at 2880 cmand the Fermi resonance of this mode at 2945 cm .Besides, the symmetric stretching mode of CH2ss at 2850 cm is minor or hardly observed. The verylow intensity of CH2ss in compare to CH modes indicates that the AA monolayers have been wellformed at LC phase on the surface of solutions [3]. For convenience of discussion, we separate the spectra into two bands depicted in two panel ofeach figure, in which the CH vibrational band on the left and the OH stretching band on the right. TheOH stretching band broaden 3000 cm° to 3600 cm in the IR region. In this region, the peak at ~3450cm is assigned to OH groups hydrogen-bonded to neighbors in a relatively disordered structure.Thepeak at~3200 cmis assigned to OH groups hydrogen-bonded in a well ordered"ice-like”structure [2]. a b) Figure 2. SFG spectra of AA monolayer/ purewater surfaces. Figure 3. SFG spectra of monolayer AA/ salt solutions NaCl. We observe a general trend of decreasing SF intensities in presence of salts in the beneathsolutions, except for NaF which will be discussed later. This observation agrees with the previousstudies about surface propensities of those relevant ions [4, 5]. In the left panels of Fig. 2 and Fig. 3,we can observe a decreasing of SFG signals in the CH band in presence of NaI and NaCl in thesolutions in compare to those from the AA over pure water. Y. R. Shen et. al. [8] have used phase-sensitive SFVS to investigate surface propensities of various ions in salt solutions and found adecreasing trend in surface propensities of the ions in following order: I, Cl, and Na [4]. Moleculardynamic (MD) simulations have also predicted similar results [5]. In our experiment, the decreasing of SF signals in the CH band indicates that the presence ofI ,Cl at the interfaces disturbs the well-order network of AA molecules of the monolayer on the water.In the meantime, anions I, Clalso disturb the hydro-bonding network of the AA’s head-groups withthe water molecules at the interfaces, leading to the decreasing of SF intensities in the ice-like band (~3200 cm’') as seen in Fig. 2b, and Fig. 3b. For more detail, we compare effect of I, Cl ions on the interfacial structures of the AAmonolayer on solutions of NaI and NaCl with the same salt concentration of 1M, as indicated in Fig.4. As seen in Fig. 4a, the peak intensities of CH3ss and CH3Fr from the AA monolayer on the NaI (1M)solution are lower than that from the AA on the NaCl (1M) solution. This difference can be explainedby the larger size and more polarizability ofI anions in compare to those of the Cl, or the I ionshave a larger surface propensity in relative to the Cl. In Fig. 4b, we observed a reversed trend of effects ofI and Cl on the OH vibrational band incompare to those on the CH band. The SF intensity of OH “ice-like”vibrations (~ 3200 cm) in theinterface ofNaI solution is larger than that from the interface of NaCl solution. For this observation,we propose an explanation as following: due to their higher surface propensity,I ions “emerge”fromthe top-most interfacial layers with a larger number in compare to C1ions, this point turn out to beagreed with a recent MD simulation result [5]. Thus, these I ions will have a larger effect on the AAmolecular network, and the Cl ions, on the other hand, disturb the ice-like structure of the waterinterfacial layers below leading to a decreasing of the SF signal from this structure. Figure 4. SFG spectra of monolayer AA/ salt solutions NaI, NaCl at the same concentration. We observe a contrary effect of F on SF spectra from AA monolayer on NaF solutions. In Fig.5a, the peak intensities of CH3ss and CH3Fr are almost unchanged in presence of NaF in the solutions.with concentration ranging from 0 to 0.8M (notice that the saturation concentration of NaF is ~1M).However, the SF intensities from OH“ice-like”bands are significantly increased in the presence ofNaF in solutions, as seen in Fig. 4b. Results from molecular dynamic studies of Pavel Jungwirth et. al[5] predicted that F ions in the solution submerge in the top-most water interfacial layer. These ionsinteract with water molecules at the interfacial layers enhancing the hydrogen bonding network ofwater at the interface. This result is also in agreement with those from Richmond et. al.[6], whichshowed that F anions do not emerge from the topmost layer of an air/water interface and have a“structure-making”characteristic that enhances the water network at the interface. Figure 5. SFG spectra of monolayer AA/ salt solutions NaF. 4. Conclusions In this paper, we use sum-frequency generation vibrational spectroscopy to study effects of varioushalide anions in the AA Langmuir monolayer on water interface. The results show that those anions affect the interface in different ways. BothI and Cl ions have high surface propensities and disturbstructures at the interface, but due to its higher surface propensity, the I shows more effect on themonolayer structure, whereas the C1 shows more effect on the “ice-like”structure of the beneathwater. The presence of fluoride (F ), on the other hand, shows an enhancement ofthe hydrogenbonding of water at the interface. These results reinforce similar arguments of some recent studiesobtained by molecular dynamic simulation as well as by phase-sensitive SFG-VS. References ( [1] D . M yers, S u rfaces, Interfaces, and C olloids: P rinciples and A pplications, Wiley-VCH Pub l ishers, New York,1999. ) ( [2] Du, Q.; S uperfine, R.; Freysz, E .; Shen, Y. R ., Vibrational S pectroscopy of Wa t er at the Vapor/Water Interface,Physical Review Letters 7 0 (1993) 2313. ) ( [3] P.Guyot- Sionnest, J.H.Hunt, and Y . R.Shen, Sum- Frequency Vibrational Spectroscopy of a Lang m uir Film: Study of Molecular Orientation of a Two Dimensional System, Physical Review Letters 59 (1987)1597. ) ( [4] Chuanshan T i an, S t even J . Byrnes,Hui-Ling Han, an d Y. Ro n She n , Surface Propensities of Atm o sphericallyRelevant I ons i n SaltSolutions Revealed by P hase-Sensitive Sum F r equency VibrationalSpectroscopy, JournalPhysical Chemistry L e tters 2 (2011 ) 1 9 46. ) ( [5] Pavel J ungwirth, a nd Douglas J . Tobias, Ions at the A i r/Water In t erface, Journal P h ysical Chemistry B 106(2002) 6361. ) ( [6] E lizabeth A. Raymond and Geraldine L. R i chmond, Pr o bing the Molecular St r ucture and Bonding of the Surfaceof Aqueous Salt Solutions, Journal Physical Chemistry B 108 (2004) 5 051. ) In this report, we studied the effect of halide salt concentration on Arachidic Acid (AA) Langmuir monolayers formed on salt solutions, such as NaCl, NaI, NaF by using a Sum-Frequency Generation Vibrational Spectroscopy. In the SFG spectra, we observed the peak intensities of CH3FR and CH3SS vibrational modes decreased when increasing the salt concentration. This observation indicates that the structure of the AA monolayer was disordered by the interaction of the dissolved ions with the carboxyl head groups of the monolayer.
关闭-
1/6

-
2/6
还剩4页未读,是否继续阅读?
继续免费阅读全文产品配置单
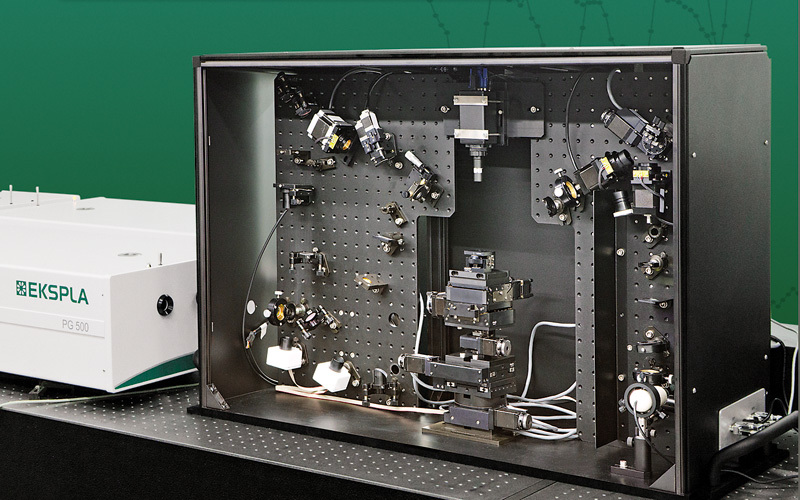
北京欧兰科技发展有限公司为您提供《花生酸和盐溶液中振动和频光谱(SFG)检测方案(其它光谱仪)》,该方案主要用于无机酸中可靠性能检测,参考标准《暂无》,《花生酸和盐溶液中振动和频光谱(SFG)检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统、PG500 系列二倍频泵浦的皮秒光学参量发生器(OPG)。
我要纠错