方案详情文
智能文字提取功能测试中
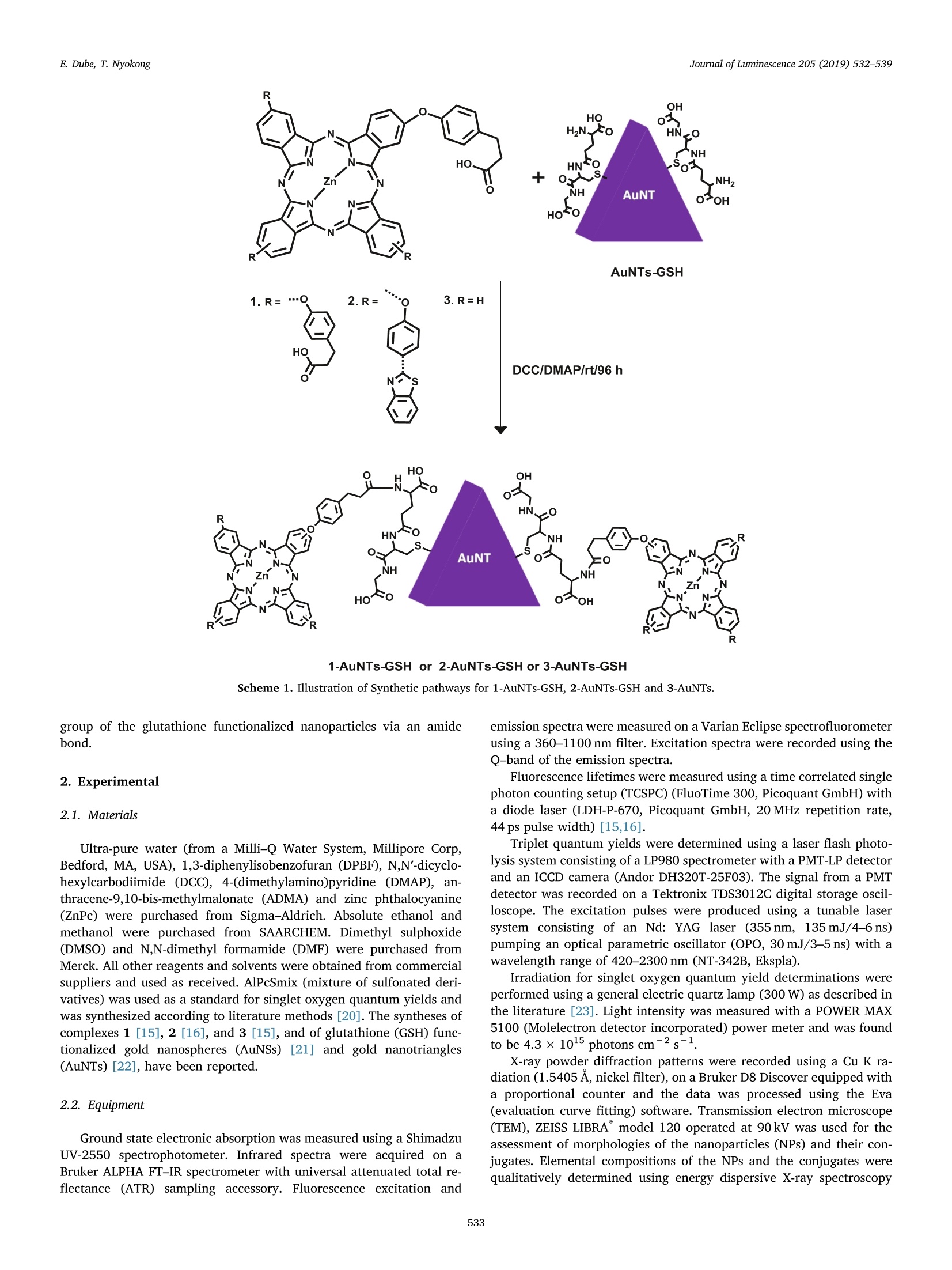
Journal of Luminescence 205 (2019) 532-539Contents lists available at ScienceDirectJournal of Luminescence E. Dube, T.NyokongJournal of Luminescence 205 (2019) 532-539 journal homepage: www.elsevier.com/locate/jlumin Full Length Article Effect of gold nanoparticles shape and size on the photophysicochemicalbehaviour of symmetric and asymmetric zinc phthalocyanines Edith Dube, Tebello Nyokong* Center for Nanotechnology Innovation, Department of Chemistry, Rhodes University, Grahamstown 6140, South Africa A RTICLEIN F O A BSTRACT Keywords: Glutathione (GSH) capped Au nanotriangles (AuNTs-GSH) and nanospheres (AuNSs-GSH) are covalently linkedGold nanotriangles to symmetric Zn phthalocyanine (ZnPc) substituted with phenoxy propanoic acid substituents only (complex 1)Nanospheres and two asymmetric ZnPc, each containing one phenoxy propanoic acid and three benzothiazole phenoxyTriplet quantum yield moieties (complex 2), and one phenoxy propanoic acid and no other ligands (complex 3). The photo-Zinc phthalocyaninesphysicochemical behaviour of Pc complexes and their conjugates were studied. All conjugates displayed im-Asymmetryproved triplet and singlet oxygen quantum yields with decreases in fluorescence quantum yields compared totheir respective Pc complexes. The conjugates of asymmetric complexes 2 and 3, afforded much higher tripletand singlet oxygen quantum yields compared to the symmetric complex 1, and could serve as good candidatesfor photodynamic therapy. 1. Introduction Metallophthalocyanines (MPcs) are dyes that have found use asphotosensitisers (PS) in applications such as photodynamic therapy(PDT) [1-3],photodynamic antimicrobial chemotherapy (PACT)[4-6],photodegradation of pollutants [7] and nonlinear optics [8]. The pre-sence of heavy atoms in the central cavity of Pcs promotes intersystemcrossing to a triplet state [9]. The triplet state reacts with the groundstate molecular oxygen via energy transfer to form singlet oxygen andother reactive oxygen species that can destroy cancer cells and patho-genic microbes. The efficiency of singlet oxygen generation of a PS isimportant in applications such as in PACT, PDT and photodegradationofpollutants [10]. Selective accumulation of the PS to cancerous cells is paramount inPDT applications [11,12], and as a result, studies on the use of nano-carriers are on the increase. Gold nanoparticles have been linked toMPcs as nanocarriers for improved targeting through the enhancedpermeability and retention (EPR) effect [13]. The attractiveness of goldas a nanocarrier emanates from its biocompatibility, inertness and non-toxicity within biological systems [14]. Its ability to improve the pho-tophysical and photochemical properties of MPcs through the heavyatom effect is an added advantage. This work reports on the covalent linkage of symmetric (complex 1)and asymmetric (complexes 2 and 3) zinc phthalocyanines to glu-tathione functionalized gold nanotriangles(AuNTs-GSH) and nanospheres (AuNSs-GSH). Zinc phthalocyanines are employed in thiswork since they are known to possess high triplet and singlet oxygenquantum yields. We then compare the photophysical behaviour of thegold conjugates of the symmetric ZnPc (with phenoxy propanoic acidsubstituents only, complex 1) and those of two asymmetric ZnPc, eachcontaining one phenoxy propanoic acid and three benzothiazole phe-noxy moieties for complex 2, and no other groups for complex 3.Porphyrins containing propanoic acid (such as the Uroporphyrins) havebeen successfully applied for PDT [2], hence the interest in Pcs con-taining propanoic acid substituents, additionally Pc complexes con-taining phenoxy propanoic acid displayed reasonably high triplet andsinglet oxygen quantum yield [15,16]. The covalent linkage of goldnanotriangles to phthalocyanines is reported for the first time and thisis further compared with covalent linkage of the correspondingphthalocyanines with gold nanospheres. The conjugates of phthalo-cyanies (Pc) with gold spheres (AuNS) have been compared with thoseof rods, stars and bypyramids [17], however we compare for the firsttime the conjugates of spheres with those of triangles. Gold nano-triangles were reported to display better cellular uptake compared toother anisotropic nanoparticles (order of cellular uptake efficiency:triangles > rods > stars [18]), while on the other hand a simulationmodel of gold nanoparticles demonstrated the highest uptake of na-nospheres compared to rods, cubes and disks [19]. An efficient cellularuptake is good for applications in photodynamic therapy. For thecovalent linkage, the carboxylic acid of the Pcs was linked to the amino ( E-mail address: t .nyokong@ r u .ac. z a ( T. Nyokong). ) ( h t t p s :// doi . org / 1 0.1016/ j . j l u min.20 1 8 .09.063 ) ( Received 19 July 2018; Received in revised form 1 0 September 2018; Accepted 28 September 2018 ) ( Available online 01 October 2018 ) ( 0022-2313/ C 2018 Elsevier B.V. All r i ghts reserved. ) R 1-AuNTs-GSH or 2-AuNTs-GSH or 3-AuNTs-GSH Scheme 1. Illustration of Synthetic pathways for 1-AuNTs-GSH, 2-AuNTs-GSH and 3-AuNTs. group of the glutathione functionalized nanoparticles via an amidebond. 2. Experimental 2.1. Materials Ultra-pure water (from a Milli-Q Water System, Millipore Corp,Bedford, MA, USA), 1,3-diphenylisobenzofuran (DPBF), N,N'-dicyclo-hexylcarbodiimide (DCC), 4-(dimethylamino)pyridine (DMAP), an-thracene-9,10-bis-methylmalonate (ADMA) and zinc phthalocyanine(ZnPc) were purchased from Sigma-Aldrich. Absolute ethanol andmethanol were purchased from SAARCHEM. Dimethyl sulphoxide(DMSO) and N,N-dimethyl formamide (DMF) were purchased fromMerck. All other reagents and solvents were obtained from commercialsuppliers and used as received. AlPcSmix (mixture of sulfonated deri-vatives) was used as a standard for singlet oxygen quantum yields andwas synthesized according to literature methods [20]. The syntheses ofcomplexes 1 [15], 2 [16], and 3[15], and of glutathione (GSH) func-C-tionalized gold nanospheres (AuNSs) [21] and gold nanotriangles(AuNTs) [22], have been reported. 2.2. Equipment Ground state electronic absorption was measured using a ShimadzuUV-2550 spectrophotometer. Infrared spectra were acquired on aBruker ALPHA FT-IR spectrometer with universal attenuated total re-flectance (ATR) sampling accessory. Fluorescence excitation and emission spectra were measured on a Varian Eclipse spectrofluorometerusing a 360-1100 nm filter. Excitation spectra were recorded using theQ-band of the emission spectra. Fluorescence lifetimes were measured using a time correlated singlephoton counting setup (TCSPC) (FluoTime 300, Picoquant GmbH) witha diode laser (LDH-P-670, Picoquant GmbH, 20 MHz repetition rate,44 ps pulse width) [15,16]. Triplet quantum yields were determined using a laser flash photo-lysis system consisting of a LP980 spectrometer with a PMT-LP detectorand an ICCD camera (Andor DH320T-25F03). The signal from a PMTdetector was recorded on a Tektronix TDS3012C digital storage oscil-loscope. The excitation pulses were produced using a tunable lasersystem consisting of an Nd: YAG laser (355 nm, 135 mJ/4-6 ns)pumping an optical parametric oscillator (OPO, 30 mJ/3-5 ns) with awavelength range of 420-2300 nm (NT-342B, Ekspla). Irradiation for singlet oxygen quantum yield determinations wereperformed using a general electric quartz lamp (300 W) as described inthe literature [23]. Light intensity was measured with a POWER MAX5100 (Molelectron detector incorporated) power meter and was foundto be 4.3 ×1015 photons cm-2s-1. X-ray powder diffraction patterns were recorded using a Cu K ra-diation (1.5405 A, nickel filter), on a Bruker D8 Discover equipped witha proportional counter and the data was processed using the Eva(evaluation curve fitting) software. Transmission electron microscope(TEM), ZEISS LIBRA" model 120 operated at 90kV was used for theassessment of morphologies of the nanoparticles (NPs) and their con-jugates. Elemental compositions of the NPs and the conjugates werequalitatively determined using energy dispersive X-ray spectroscopy R 1-AuNSs-GSH or 2-AuNSs-GSH or 3-AuNSs-GSH Scheme 2. Illustration of Synthetic pathways for 1-AuNSs-GSH, 2-AuNSs-GSH, and 3-AuNSs-GSH. 2.3. Linkage of complexes 1 and 2 to AuNTs-GSH (Scheme 1) and AuNSs-GSH (Scheme 2) AuNTs [22] and AuNSs [21] were synthesized and functionalizedwith glutathione as reported in the literature. The Pc complexes werecovalently linked to the NPs to form conjugates as follows: complex 1(0.021g, 0.017 mmol), complex 2 (0.023g, 0.016mmol), and 3(0.012g, 0.016 mmol) were separately dissolved in 2 mL of dry DMF.The coupling agents, DCC (0.01 g, 0.049 mmol) and DMAP (0.005 g,0.042 mmol) were added to activate the carboxylic acid group of the Pccomplexes to allow for covalent linkage to NPs via amide bond for-mation. The reaction mixtures were stirred for 48 h at room tempera-ture after which 0.05 g of AuNTs-GSH was added and the reactionmixture was further stirred for 48 h. The same protocol was employedfor AuNSs-GSH. The conjugates (1-AuNTs-GSH, 1-AuNSs-GSH, 2-AuNTs-GSH, 2-AuNSs-GSH, 3-AuNTs-GSH, and 3-AuNSs-GSH) wereprecipitated out with methanol and centrifuged. The precipitates werepurified by washing with ethanol (with centrifuging) and allowed todry in the fume hood. Fluorescence (Dp) and triplet (r) quantum yields of Pc complexesand the conjugates were determined in DMSO using comparativemethods described before in literature [24,25]. Unsubstituted ZnPc inDMSO was used as a standard with Dp = 0.20 [24] andr=0.65[26].The solutions for triplet state studies were de-aerated with argon for15 min before measurements. Singlet oxygen quantum yield (DA) values were determined underambient conditions using DPBF as a singlet oxygen quencher in DMSO(and ADMA in water) and equations described before [27,28]. ZnPc inDMSO was used as a standard (A(Std)= 0.67 in DMSO) [27]. AlPcSmixwas employed as a standard in aqueous media (PA(Std) =0.42 [25]).The absorbances of DPBF or ADMA were spectroscopically monitored at417 nm or 380 nm, respectively, at predetermined time intervals. 3. Results and discussion 3.1. Characterization of NPs and conjugates Schemes 1 and 2 show the covalent linkage of Pc complexes toAuNTs-GSH and AuNSs-GSH respectively via an amide bond betweenthe carboxylic acid of the propanoic acid substituents and the aminogroup of glutathione in glutathione functionalized gold nanoparticles.Complex 2 also has a benzothiazole substituent which contains sulphur,known for its affinity for gold. It should however be noted that the Fig. 1. UV-vis absorption of (A) AuNSs-GSH (a), AuNTs-GSH(b), (B) complex 1 (a), 1-AuNSs-GSH (b), 1-AuNTs-GSH (C), (C) complex 2 (a), 2-AuNSs-GSH (b), 2-AuNTs-GSH (c), (D) complex 3 (a), 3-AuNSs-GSH (b), 3-AuNTs-GSH (c) in DMSO. Table 1Photophysicochemical parameters of complexes 1, 2 and their conjugates in DMSO. Samples Size (TEM)(nm) Pc loading (ug/mg) Xabs(nm) PF(±0.01) F(ns) (±0.01) PT(±0.01)(DMSO) TT(us) (±1.00) PA(±0.01) b 1 一 680 0.19 3.36 0.54 264 0.47(0.07) 1-AuNTs-GSH 34.9(33.2) 22 680 0.13 3.05 0.68 244 0.59(0.14) ‘1-AuNSs-GSH 14.9(13.6) 27 679 0.03 2.87 0.70 305 0.58(0.15) 2 - 680 0.15 2.86 0.73 238 0.69 (0.15) 2-AuNTs-GSH 37.1(33.2) 30 681 0.08 2.30 0.83 229 0.75(0.20) 2-AuNSs-GSH 19.8(17.7) 37 679 0.06 2.24 0.90 216 0.79(0.22) 3 675 0.18 3.21 0.73 233 0.55(0.12) 3-AuNTs-GSH 36.9(33.2) 16 673 0.06 3.09 0.80 247 0.69 (0.17) 3-AuNSs-GSH 13.8(13.6) 20 674 0.02 2.98 0.87 262 0.72 (0.23) a Sizes as edge length for AuNTs-GSH and diameter for AuNSs-GSH; numbers in brackets are the sizes in nm for AuNTs-GSH or, AuNSs-GSH alone.numbers in brackets are the values in water and for complexes alone (1, 2 and 3) the values are in water containing 0.5% DMSO. Literature values for 1 and 3 [15], for 2 [16]. presence of the capping agent, GSH, reduces the Au-S interactions. Atthe same time, the activated carboxylic acid moieties of 2 are expectedto interact with the NH2 group of GSH faster than the possible inter-action of sulphur in benzothiazole with Au in glutathione functiona-lized gold nanoparticles. Though the Pc complexes alone are not water soluble, the presenceof glutathione in nanoparticles makes the conjugates readily soluble inwater, which is important for practical applications. However, singletoxygen determinations of the Pc complexes alone in water were carriedout using 0.5% DMSO in water. 3.1.1. Electronic Absorption and emission spectra of complexes and theirconjugates Fig.2. Emission (a), excitation (b) and absorption (c) spectra of 2-AuNTs-GSH(excitation = 609 nm, solvent = DMSO). The surface plasmon peaks of AuNTs-GSH and AuNSs-GSH (Fig.1)were observed at 607 nm (dipole peak) and 532 nm, respectively. TheAuNTs-GSH also displayed a shoulder peak at 600 nm, and this couldbe attributed to the presence of another size (smaller) of nanotriangles. Fig. 3. FTIR spectra of AuNTs-GSH, 1-AuNTs-GSH and complex 1. Fig. 4. XRD diffractograms for AuNSs-GSH, 1-AuNSs-GSH and 1-AuNTs-GSH. The electronic absorption spectra of 1, 2 and 3 and their conjugateswith both AuNTs-GSH and AuNSs-GSH are shown in Fig. 1. The Qbands for 1 and 2 are red shifted compared to 3,Table 1. For complex 2the red-shifting is due to the presence of S and N atoms which areknown to red-shift the Q band [23]. The Pc complexes alone displayminimum to no absorption between 400 nm and 600 nm; however,upon linkage to AuNPs there was enhancement of absorption especiallybetween the 400-650 nm regions, confirming successful linkage. Thelinkage of AuNSs-GSH to complexes 1 and 2 red shifted the SPR peakfrom 532 nm to 535 nm (Fig. 1B(b)) and 549 nm (Fig. 1C(b)), for 1-AuNSs-GSH and 2-AuNSs-GSH, respectively,while 3-AuNSs-GSH wasblue shifted at 530 nm (Fig. 1D(c)). The red shifting is usually asso-ciated with an increase in size of the nanoparticles after linkage to Pc complexes [21],however it should be noted that aggregation can alsolead to an increase in size. The SPR peaks for 1-AuNTs-GSH, 2-AuNTs-GSH, and 3-AuNTs-GSH overlapped with the vibronic band of thephthalocyanines, hence the peaks are not clear. In water (Fig S1B,ESIt), the conjugates display broad bands due to aggregation typical ofPes in aqueous solution [29] resulting from s-st electron interaction ofthe aromatic rings of Pcs. The loading of complexes 1 to 3 onto the nanoparticles was in-vestigated following literature methods [30]. This involves comparingthe Qband absorbance intensity of the Pc in the conjugate with that ofthe initial Pc before the conjugation. The loadings of Pc onto nano-particles are listed in Table 1. There is less loading for the AuNTscompared to AuNSs. Nanotriangles (also termed nanoplates) have atendency to stack on each other face-to-face or edge to edge [31], andthis could be the reason for less loading compared to nanospheres. The emission, excitation and absorption spectra of 2-AuNTs-GSH (asan example) are shown in Fig. 2. The emission spectra were mirrorimages of the excitation spectra. The ground state electronic absorptionand excitation spectra of Pes are usually similar, however slight dif-ferences are observed in the conjugates probably due to the absorbanceby the AuNPs. 3.1.2. FTIR spectra The FTIR spectra (Fig. 3, using AuNTs-GSH, 1-AuNTs-GSH andcomplex 1, as examples) were employed to prove amide bond formationbetween the Pc complexes and the NPs. The FTIR spectrum of AuNTs-GSH exhibited characteristic primary carboamide double peaks at1529-1585 cm-and 3260-3313 cm-which changed to single (sec-ondary carboamide) peaks at 1540 cm- and 3248 cm-1, respectivelyon linkage of AuNTs-GSH to complex 1, confirming the formation of anamide bond between glutathione functionalized NPs and the Pc com-plexes. Also observed was the disappearance of peaks at 1706 cm-and1603cm-1 in complex 1 and 1629 cm-1 in AuNTs-GSH. Shifts andchanges in the IR bands confirm structural change [32]. An amide peakwas observed at 1655 cm- after conjugation; however, it should benoted that GSH alone has amide bonds shown by peaks at 1654cmand 1629cm-in AuNTS-GSH. The same trend was also observed for the conjugates 1-AuNSs-GSH,2-AuNSs-GSH, 2-AuNTs-GSH,3-AuNSs-GSH and 3-AuNTs-GSH, 3.1.3. XRD studies Fig. 4 shows the powder XRD patterns of AuNSs-GSH, 1-AuNSs-GSHand 1-AuNTs-GSH, used as examples. The XRD diffraction patterns arecharacterized by well-defined crystalline peaks at 20 =38.1, 43.9,64.5, 77.4 and 81.0°, assigned to the 111, 200, 220,311 and 222planes, corresponding to the face centered-cubic structures of metallicgold [33], confirming its presence. Broad peaks between 20 = 15-23°,characteristic of the amorphous nature of phthalocyanines [34] wereobserved, which provides evidence for the presence of the phthalo-cyanines in the conjugates. 3.1.4. TEM The TEM micrographs of AuNTs-GSH, 1-AuNTs-GSH are shown inFig.5A,C, respectively,and those of AuNSs-GSH, 1-AuNSs-GSH inFig. 5B,D, respectively. The AuNTs-GSH and AuNSs-GSH are mostlymonodispersed when alone and show aggregation after linkage to Pcs.Aggregation is common in the Pc-NP conjugates, and is mostly attrib-uted to -s stacking that can occur between the Pcs on adjacent NPs.Pcs are known for their J-jt stacking tendency to form H aggregates[29]. The average sizes of the NPs (edge lengths for AuNTs-GSH anddiameter for AuNSs-GSH) and their conjugates are displayed in Table 1.Increase in size was observed in all conjugates due aggregation as dis-cussed above. 3.1.5. EDX Theelemental compositionss of thee nanoparticles, Pcsand Fig. 5. Representative TEM micrographs for AuNTs-GSH (A), AuNSs-GSH (B), 1-AuNTs-GSH (C), and 1-AuNSs-GSH (D). conjugates were qualitatively determined using an energy dispersive X-ray spectrometer (EDX) and Fig. 6 shows the spectra for AuNTs-GSH,complex 2 and 2-AuNTs-GSH as examples. The EDX spectra for AuNTs-GSH showed the presence of C, N,O,S and Au, while complex 2 showedC, N, O, S and Zn as expected for this Pc. The spectrum for 2-AuNTs-GSH displayed C, N, O, S, Au and Zn, confirming the presence of boththe nanoparticle and the phthalocyanine. 3.2. Photophysicochemical parameters The fluorescence quantum yields (Dp) and lifetimes (tp), tripletquantum yields (Dr) and lifetimes (tr), and singlet oxygen quantumyields () of complexes 1, 2, and 3 alone, and their conjugates usingDMSO (and in water in brackets) are as displayed in Table 1. 3.2.1. Fluorescence quantum yields (Dp) and lifetimes (tp) When comparing Pcs alone, complexes 1 and 3 containing no ben-zothiazole phenoxy groups, have about the same Dp values which ishigher than for 2, suggesting quenching by benzothiazole substituentfor the latter. On covalent linkage to AuNSs-GSH and AuNTs-GSH, allPcs exhibited reduced Dp (Table 1), as expected due to the heavy atomeffect of gold, which promotes intersystem crossing to the triplet statethus reducing fluorescence. The AuNSs-GSH conjugates showed lowerD than their corresponding AuNTs-GSH conjugates, probably due tothe higher number of Pcs loaded on the former compared to the latter.The higher number of Pcs could lead to aggregation, resulting inquenching of fluorescence. Fluorescence behaviour of dyes in the presence of nanoparticles hasbeen shown to be affected by the size of the nanoparticles [35], with thesmaller NPs exhibiting a greater quenching effect. This is observed inthis work where 1-AuNSs-GSH and 3-AuNSs-GSH, with relativelysmaller size of the NSs compared to 2-AuNSs-GSH, have smaller Dp values, Table 1. This also applies when comparing the larger size ofAuNTs-GSH (33.2nm-edge length) with the smaller AuNSs-GSH (17.7/13.6 nm diameter), where there is more quenching of Dp for complexesin the presence of the latter. The conjugates displayed a bi-exponential decay, hence existence oftwo lifetimes (Fig. 7, using 3-AuNTs-GSH as example), possibly due todifferent orientations of the phthalocyanines on the NPs [36]. Theaverage lifetimes for the conjugates are presented in Table 1. Thefluorescence lifetimes decreased with the decrease in Dp since the twohave a direct relationship. 3.2.2. Triplet quantum yields (Dr) and lifetimes (rT) The Pr greatly influences the singlet oxygen production, thus highDr, corresponding to low Dp, signal a more efficient intersystemcrossing, an attractive feature for photosensitizers. The triplet decayand transient curve of the conjugate 1-AuNTs-GSH,( as an example) areshown in Fig. 8. The transient curve is characterized by a broad bandbetween 380 and 600 nm with a peak at 500 nm, attributed to the tri-plet-triplet state excited absorption (T1→Tn). The negative peaks shownaround 358nm and 681 nm are attributed to the depletion of thephthalocyanine's ground state [37]. The triplet decay curve obeyedsecond order kinetics, typical of MPc complexes at high concentration,due to triplet-triplet recombination [38]. Comparing Pcs alone, theasymmetrical complexes 2 and 3 show a much larger Dr values com-pared to 1. Asymmetry is known to introduce distortions [39], resultingin faster intersystem crossing to the triplet state, consequently in-creasing the triplet state population. The conjugates of Pcs with NPsgenerally displayed further increased Dr (Table 1) than Pc complexesalone, corresponding to the low Dp, due to the presence of gold, a heavyatom as explained before. AuNTs-GSH conjugates displayed lower Drvalues than their corresponding AuNSs-GSH counterparts. The lower Drof AuNTs-GSH conjugates was attributed to less loading which probably AuNTs-GSH 0 2 5 6 9 10 Fig. 6. EDX spectra of AuNTs-GSH, complex 2, and 2-AuNTs-GSH. Fig.7. Fluorescence decay (blue), x"fitting (red) and IRF (black) curves for 3-AuNTs-GSH in DMSO. was influenced by size and the tendency of nanotriangles to stack oneach other as explained before. The Dr of the conjugates in water couldnot be obtained due to aggregation of phthalocyanines in water. 3.2.3. Singlet oxygen quantum yields Singlet oxygen is produced when the PS in the excited triplet statetransfers its energy to the molecular oxygen. The D parameter showsthe efficiency of singlet oxygen production. For the determination ofthe singlet oxygen quantum yield (D), the chemical photodegradation Fig. 8. (A)Transient curve and (B) triplet absorption decay curve (black) andfitting (red) for 1-AuNTs-GSH in DMSO. Fig. 9. Representative spectra for singlet oxygen quantum yield determinationusing a photochemical method. The spectra show the degradation of (A) DPBFin DMSO and (B) ADMA in water in the presence of 2-AuNTs-GSH. of singlet oxygen quenchers (DPBF in DMSO and ADMA in aqueousmedia (using 2-AuNTs-GSH as an example)) were monitored over aperiod of time (Fig. 9). The Q-band remained unchanged, proving theirstability over the irradiation period, while the DPBF and ADMA bandsdegraded. The followed the same trend observed in r since the value isdependent on the Dr parameter, as previously stated. It should be notedthat for studies in aqueous media, water alone was used for the con-jugates since they are readily soluble as explained before, but for the Pccomplexes alone which are insoluble in water, 0.5%(v/v) DMSO inwater was used. Due to aggregation, the values in water are low how-ever they still maintained the trend regardless of the slight differencesin the solvent system used. PS with low D such as lutetium texaphyrin(_=0.11) have been employed for clinical application in PDT [2],hence the D values for all conjugates shows that they may be used forPDT applications in the presence of both spherical and triangularAuNPs. 4. Conclusion In this work, the covalent linkage of AuNTs-GSH and AuNSs-GSH tocomplexes 1 to 3 via an amide bond is reported. The conjugates formedwere characterized using UV/vis absorption and emission spectrometer,FT-IR spectrometer, XRD, TEM and EDX. The photophysicochemicalbehaviour of complexes and their conjugates were studied. The linkageof complexes with nanoparticles resulted in a decrease in fluorescencequantum yields and lifetimes, which led to improved triplet and singletquantum yields. Even though conjugates with spherical NPs displayedbetter properties than triangular NPs, all conjugates are ideal for PDTapplications. Acknowledgements This work was supported by the Department of Science andTechnology (DST) Innovation and National Research Foundation(NRF), South Africa through DST/NRF South African Research ChairsInitiative for Professor of Medicinal Chemistry and Nanotechnology(UID 62620) as well as Rhodes University. Appendix A. Supplementary material Supplementary data associated with this article can be found in theonline version at https://doi.org/10.1016/j.jlumin.2018.09.063. ( References ) ( [ 1] L . M . M o r eira, F . V . d os S a n tos, J. P . L yon, M. Maf t oum -Co sta, C . P a c h eco -S o ares, N . S . d a S ilv a, P h ot o dyn am ic t h er ap y : p orph y rins a n d p htha l ocy an i n e s as ph ot o-s e n s i t iz e r s, Aust . J . Ch e m. 6 1 (2008) 7 41 -7 5 4. ) ( [2] R . B o n n et t , I n C hemi c a l As p ec t s of Photod y n a mi c The r ap y , Gordo n a nd B r e ach S ci ence P ub l ish er s , A mste r da m , 2 0 00. ) ( [3] S . B . Bro w n , E. A . B rown , I. Wa l ker, T he p re s e n t an d f utu r e role of photod yn amic t h e ra py i n c a nc e r t re a t m ent, L a nce t O ncol. 5 ( 20 04) 4 9 7-50 8 . ) ( [4 ] V . N . M a nt a reva, I . A nge lo va, D. W ohrleb, E . B oris o va c , V . K u ss o v ski, ) ( M eta l lophth a l o c y an i n e s as p h otody n a m ic se n s i t izer s for tr e a t m en t of p a t h o g enic b D a c te r i a . U p ta k e a n d pho t oinac ti va t ion p r o per t i e s , J . Po r phyr . P h th al o c y a n i nes 1 7 ( 2013) 400 -4 16 . ) ( [5] D . M onda l , S. B er a, P orphy ri ns a n d ph t h a l o c y ani n e s: p r o misin g mo l e c ule s fo r l i g h t - t rig g ered a ntibacte r ia l nan op articles , Adv . N a t. S c i . : Na n os ci . Na n ote c h n ol . 5 ( 2 014) 0 33 002 ( 12 p ages) . ) ( [6] ] M . W ainw r i gh t , P ho t o d y na m i c a nti m i cr o b i a l c h e m o ther a p y ( P AC T) , J . A n t i mi cro b . C hemothe r . 4 2 (1998) 1 3- 28 . ) ( [7] ] 1 R . Z ug le, T e be llo N yo k o n g , C omp a ra tive ph ot otr a ns f or mat io n of en vi r o n m en ta l p ollutants u sing m etallop h thalocy an ines supported on e l e c tro spu n p o l ymer f ib e rs, J . A p p l. Pol ym . S ci. 12 8 ( 2 0 13) 1 1 3 1 - 1 142 . ) ( [8] ] M . Han a ck , T . S c h n ei d e r , M . B a r t hel , J.S . S hirk , S . R . F lom, R . G . S . Pong , I ndiu m p ht h a l ocy an ines a nd n aphth a l o cya ni nes fo r optic al l i m i t i n g , Coo r d. C h em. Re v. 219 ( 2 001 ) 23 5 - 258. ) ( [9] H . S ai g u s a , T . A zum i , M . S u m ita n i, K . Yoshiha ra, I nternal heavy a tom e f f e ct on the t r i p let s p in s u b l e ve ls o f t h e l owes t t r i plet s t at e o f na p hthale n e . I I . Int e r sy ste m c ross i ng p ro cess es f r om the s in gle t ex c i t e d s t ate t o t h e i n d ivi d ua l spin s ubleve ls o f t h e l owe st t r i pl e t s tate , J . Ch e m. Ph y s. 7 2 ( 1 980 ) 1 7 1 3 - 1 7 1 5. ) [10] M.C. DeRosa, R.J. Crutchley, Photosensitized singlet oxygen and its applications,Coord. Chem. Rev. 233-234(2002) 351-371. [11]1L.B. Josefsen, R.W. Boyle, Unique diagnostic and therapeutic roles of porphyrinsand phthalocyanines in photodynamic therapy, Imaging Thera. Thera. 2 (2012)916-966. ( [12] . J . Ta que t , C. Froch ot , V . Ma n neville , M. B a r b e r i - He y o b , P hthal o cyan i ne s c ov a l e nt l y b ou n d t o b i o m olecule s f o r a t a r ge t e d p h o t o d yn a mic th er apy , C u r r . M e d. C hem . 1 4 ( 2 0 07) 1 6 73-16 8 7. ) ( [13] Y . O m i di, J . B ar ar, T a r g e ting t u mor mic roenviron m ent : cros s i n g t um or i nter s t i ti al f lui d by mult i func ti o na l nanom e d i ci n es , B io I mpact s : B I 4 ( 20 1 4 ) 5 5- 6 7 . ) ( [14] D 1 . C . H one, P . I . W a lk er , R . E v an s-Go wi ng, S. F i t z Ge rald , A . Be e b y , I. C h a mb ri er, M . J . C o ok, D.A. R uss e l l , G e nera t i o n o f c yt o t o xi c si n gl e t o x yge n v ia p h th a lo c y an ine - s t ab i l i z e d g o l d n a n o pa rt ic l e s: a p o t e n t i a l d e live r y v e hi c l e f or p h o to d yn a mic t hera p y , Lan g mui r 1 8 ( 2 002) 2985- 2 987. ) [15]E1. Dube, N. Nwaji, D.O. Oluwole, J. Mack, T. Nyokong, Investigation of photo-physicochemical properties of zinc phthalocyanines conjugated to metallic nano-particles, J.Photochem. Photobiol. A: Chem. 349 (2017) 148-161. 111wole F[16]E. Dube, D.O. Oluwole, E. Prinsloo, T. Nyokong, Gold-chitosan composite with lowsymmetry zinc phthalocyanine for enhanced singlet oxygen generation and im-proved photodynamic therapy activity, New J. Chem. 42 (2018) 10214-10225. ( [17] T . M the t h w a,T. Ny o k o ng, F l uorescenc e b e h a v i o r a n d s i n gl et o x yg en g e ne r a t ing a b i l i ties of al um inu m p ht h a l ocy an in e i n t h e p r e s e nce o f a n is o trop i c g ol d nano- p a r ticles, J . Lu m in . 1 57 ( 201 5 ) 207 - 21 4 . ) ( [18] X . X ie, J. Li ao , X . Shao , Q. Li , Y . L i n, T he Ef fe ct of shap e on C e llu l a r U ptak e o f G old N a n o part i c l es i n t h e f o r m s o f S tar s , R o dsTr i ang l es S c i . Re p . 7 (2 01 7 ) 3 8 2 7 . ) [19] Y. Li, M. Kroger, W.K. Liu, Shape effect in cellular uptake of PEGylated nano-particles: comparison between sphere, rod, cube and disk, Nanoscale 7 (2015)16631-16646. [20]M. Ambroz, A. Beeby, A.J. McRobert, M.S.C. Simpson, R.K. Svensen, D. PhillipsPreparative, analytical and fluorescence spectroscopic studies of sulphonated alu-minium phthalocyanine photosensitizers, J. Photochem. Photobiol. B: Biol. 9(1991)87-95. ( [ 21]N . M a si l e la , T. N yok o n g, C o n ju ga te s o f low-s y mm e t ry Ge, Sn a n d Ti ca r bo x y p h t halo c ya n i n e s w i t h g lu tathio n e c a p e d g o ld na n op a r tic l es : an in v es ti gat io n of p h o t o p h y si c al b e hav io ur, J. P h o t o c hem. P h o tobi o l. A: C hem. 2 2 3 ( 2011 ) 124 - 131. ) ( [22] L . C he n , F. J i , Y . X u , L . H e, Y. M i , F . B a o , B . S u n, X . Z h an g , Q . Zha n g, H i g h - y i e l d s e e dless sy nt hesis o f triangula r g o ld nanoplat e s t hr oug h o xi d ativ e e t ch i n g , Nano L e tt . 1 4 ( 2 0 14) 72 01-7 2 06. ) ( [23] N . M a silela ,T . N y o k on g , T he in te rac t io n o f silv e r n anop a rt i cle s w i t h low s ymm e try c ys te i nyl m etallophthalo c ya n i n e s a nd t h e i r a nt i mic r o b i a l eff e c t , J . Ph o to c h e m . P h otobiol. A: Chem . 255 (2013) 1 - 9 . ) ( [24 ] A . O gunsipe, J .Y. C hen , T . N yo k o n g, P h otoph y si c al and ph o tochem i c a l st udi es of z i nc ( I I) ph t ha l o cy a n ine d er iv a t i ve s- e f f e cts of s u b stitue n t s a n d s olv e n t s , N e w J . Che m . 2 8 ( 2 004) 8 22- 82 7 . ) ( [25]T . N y o k on g, E . An tu nes, K. M. K a di s h , K. M . Sm i t h , R. G u i l a r d (Eds. ) , In Cha p t er t i tl e : P h otoc he m i cal a n d Phot o physi ca l P r o p e rties o f M e t allopht ha lo cy a n i n e s : Th eH a ndboo k o f P o rph y r in S c ie n ce, 7 World Sc i en t i f ic , S ingapore, 2 0 10, p p. 2 47-3 4 9 (cha p t. 3 4 ) . ) ( [26 ] T .H . Tr a n -Th i , C . Desforge, C . T h i ec , S . J . G asp ard,S i n gl et- sin gle t a n d tri p le t - t r iplet i ntr a molecul a r t r a nsfer proces s e s i n a c o v alen t l y lin k e d p orp h y r in - phth a locy a nine heterodim er , J . P hy s . Chem . 93 ( 1989 ) 1 226 - 1233. ) ( [27] N . A . K uznetsova , N .S. G re tsova , O. A . Yuzhakova , V . M . N e gri mo vsk ii , O . L. K a l iya , E . A . L u k’ y anets , N e w r e a g e n t s f o r d e t ermin a t i on o f t h e q u a n t um e f fi cie n c y of s i ng let o xyg e n g en eration i n a queous m e dia, R u ss. J. G en. Chem . 7 1 ( 2 0 01) 36-41 . ) ( [28] W . S p i l l er, H . Kli es c h, D . W ohrle, S . H a ckb a rth, B. R o d er, G . Sc h n urpfeil , S i n gl e t o xyg en q u an t u m y i el d s o f d iffer e n t photo-s e n s it i zer s i n pola r s o lv e nts and m ic el l a r s o l u t i o ns, J . P orphy r . P ht h a l oc y a n in es 2 (1998) 145-158. ) ( [29] M . J . S til l m an , T. Nyo k o n g , C . C . L e z no ff, A. B.P. L e v er (Ed s. ), P h thal o c ya ni n e s: P r operties a n d A pp l i c a tions , 1 VCH Pu b lishe r s , N e w Y ork, N Y, 1 9 8 9. ) [30]L. Li, J.F. Zhao, N. Won, H. Jin, S. Kim, J.Y. Chen, Quantum Dot - Aluminumphthalocyanine Conjugates perform photodynamic reactions to kill cancer cells viafluorescence resonance energy transfer (FRET), Nanoscale Res. Lett. 7 (2012)386-393. ( [31 ] Y . B ae, N .H . K im, M . K i m, K . Y . L ee , S . W . Han, A n isotropic as sembly o f A g n a - n o p r i sms , J . Am. Chem. Soc . 1 3 0 (2 008 ) 54 3 2-543 3. ) ( [32] B . C . Smith, I n f ra red Spec t r a I n te rpreta t i on: A Sys t e m App r o ac h , C R C P r e s s , N ew Y ork, 1998. ) [33]M.H. Majles Ara, Z. Dehghani, R. Sahraei, A. Daneshfar, Z. Javadi, F. Divsar, ( D iffrac t ion patte r ns a n d n onlinea r optica l properties o f g o ld n a noparti c l e s, J . Quant . S pec trosc. Radi a t . Tr ansf. 11 3 (2 012 ) 36 6 - 3 7 2. ) ( [34] P r a b a k a ra n R . P r aba k a ra n , R . K e sav a m o o rthy, G .L .N . Re d d y, F.P . X a v i er, S t r u ctu r a l i nve s t iga t ion of c opper p h thalocya n ine th i n f i l m s usi n g x-r a y di f f ra c t i on, ram an s c att er i ng a n d o pt ic a l a bsorpt i on me as u r em e nt s, P hys . St atus S o l i di 2 2 9 ( 2 0 02 ) 1 17 5 -11 86. ) [35]Xue, Y. Xue, L. Dai, A. Urbas, Li Quan, Size and shape-dependent fluorescencequenching of gold nanoparticles on Perylene dye, Adv. Opt. Mater. 1 (2013)581-587. ( [36] C .D. G edd e s , J .R . L a k owi c z ( Ed . ) , T opic s i n Fl u o r e s ce n c e S pe c t ro sco py, 4 03Spr i ng er, Ne w Y or k, 20 15, p p . 6 4-7 6 ( 2 005 as ci t ed i n : M . L edwab a e t a l . / J. M o l . C at al . A: C hem ) . ) ( [37] Z . N . E rol , P . Ati e nz a r , Y . A r sla n o g l u , E . H am ur yudan, H . G a r ci a , S yn t he s is a n d p h o t o p h ysica l pro p er t ies o f p h thal o cyanines h a v ing c a l ixpyrrol e units , RSC A dv . 5 (20 1 5) 5 5 9 01 . ) [38]M.G. Debacker,O. Deleplanque, B. Van Vlierberge, F.X. Sauvage, A laser photolysisstudy of triplet lifetimes and of triplet-triplet annihilation reactions of phthalo-cyanins in DMSO solutions, Laser Chem. 8 (1988) 1-11. [39]T. Fukuda, S. Homma, N. Kobayashi, Deformed phthalocyanines: synthesis andcharacterization of zinc phthalocyanines bearing phenyl substituents at the 1-, 4-,8-,11-,15-,18-,22-, and/or 25-positions, Chem. Eur. J. 11 (2005) 5205-5216. Glutathione (GSH) capped Au nanotriangles (AuNTs–GSH) and nanospheres (AuNSs–GSH) are covalently linked to symmetric Zn phthalocyanine (ZnPc) substituted with phenoxy propanoic acid substituents only (complex 1) and two asymmetric ZnPc, each containing one phenoxy propanoic acid and three benzothiazole phenoxy moieties (complex 2), and one phenoxy propanoic acid and no other ligands (complex 3). The photophysicochemicalbehaviour of Pc complexes and their conjugates were studied. All conjugates displayed improved triplet and singlet oxygen quantum yields with decreases in fluorescence quantum yields compared to their respective Pc complexes. The conjugates of asymmetric complexes 2 and 3, afforded much higher triplet and singlet oxygen quantum yields compared to the symmetric complex 1, and could serve as good candidates for photodynamic therapy.
关闭-
1/8

-
2/8
还剩6页未读,是否继续阅读?
继续免费阅读全文产品配置单
北京欧兰科技发展有限公司为您提供《酞菁锌中激光闪光光解检测方案(激光产品)》,该方案主要用于基础有机原料中机械性能检测,参考标准《暂无》,《酞菁锌中激光闪光光解检测方案(激光产品)》用到的仪器有Ekspla NT340 高能量可调谐激光器(OPO)。
我要纠错
相关方案






 咨询
咨询