方案详情文
智能文字提取功能测试中
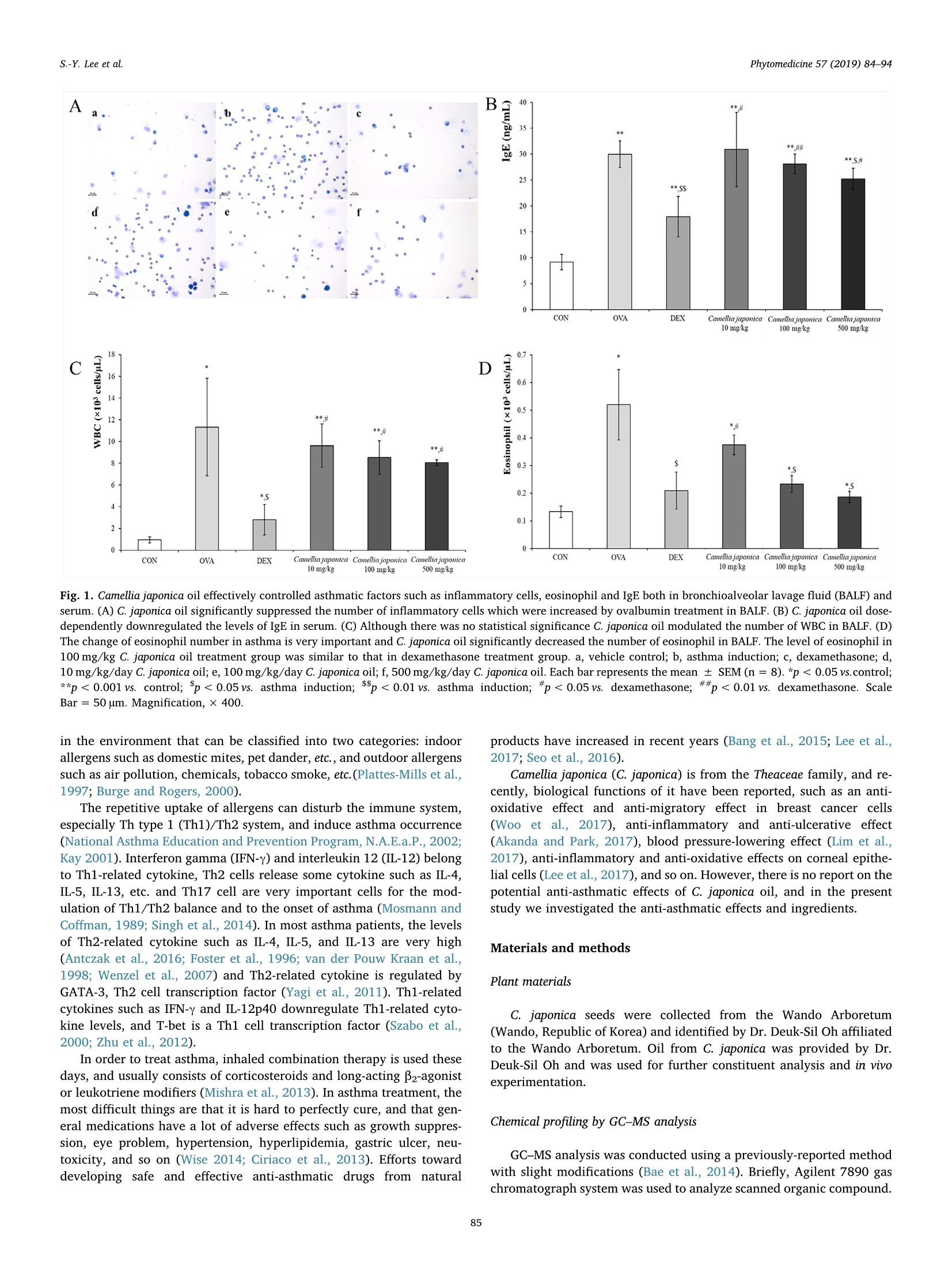
Phytomedicine 57 (2019) 84-94Contents lists available at ScienceDirect S.-Y. Lee et alPhytomedicine 57 (2019) 84-94 Phytomedicine journal homepage: www.elsevier.com/locate/phymed Original article Camellia japonica oil suppressed asthma occurrence via GATA-3 & IL-4pathway and its effective and major component is oleic acid Soon-Young Leeal, Chun-Sik Bae ,, Nam-Sook Seo, Chang-Su Na, Hah Young Yoo, Deuk-SilOh, Min-Suk Bae , Myung-Sang Kwon, Seung-Sik Cho8*, Dae-Hun Parka* College of Oriental Medicine, Dongshin University, Naju, Jeonnam 58245, South Korea 'College of Veterinary Medicine, Chonnam National University, Gwangju 61186, South Korea ‘Department of Biotechnology, Sangmyung University, Seoul 03016, South Korea Jeollanam-do Forest Resource Research Institute, Naju, Jeonnam 58213, South Korea College of Engineering, Mokpo National University, Muan, Jeonnam 58554, South Korea College of Veterinary Medicine, Kangwon National University, Chuncheon, Gangwon 24341, South Korea 8 College of Pharmacy and Natural Medicine Research Institute, Mokpo National University, Muan, Jeonnam 58554, South Korea ARTICLEINFO ABSTRAC T Keywords:C. japonica oilOleic acidTh2-related factorsOvalbumin-induced asthma model Background: In December 2016, WHO released a report stating that in 2015 there were 383,000 deaths causedby asthma and 235 million people suffering from asthma. As there are many adverse effects associated with thecurrently-used asthma drugs, new anti-asthmatic drugs need to be developed. Purpose: In order to find new drug candidates with safe and low side effects, the anti-asthmatic function andmechanism of C. japonica oil were evaluated, and its active ingredients were analyzed for use in an ovalbuminasthma murine model. Study design and methods: The study consisted of six groups: control; ovalbumin group; and dexamethasonegroup as a positive control; and 10, 100, and 500 mg/kg C. japonica oil treatment groups. In order to measure theanti-asthmatic effect of C. japonica oil, WBC and differential cell count in BALF, IgE in serum, morphologicalchanges in pulmonary system, and gene and protein levels such as IFN-y, IL-12p40, IL-4, IL-5, IL-6, TNF-a, andIL-6 were all evaluated. Results: C. japonica oil had an anti-asthmatic effect and significantly controlled eosinophil in BALF, Th2-relatedfactors such as GATA-3 that is Th2 cell transcription factor, IL-4, IL-5, and IL-13,and TNF-a in the lung. It alsodose-dependently modulated inflammatory cells, T-bet, IL-12p40, and IL-6. Oleci acid was the major gradient(52.89%) in C. japonica oil and also had anti-asthmatic effects such as the downregulation of inflammatory cells,WBC, and eosinophil in BALF, IgE in serum, and morphological changes in the lung. Conclusion: We concluded that C. japonica oil is a new anti-asthmatic drug candidate and that oleic acid is themajor anti-asthmatic ingredient in C. japonica oil. Introduction The World Health Organization (WHO) estimated that there were383,000 deaths caused by asthma in 2015, that 235 million peoplesuffered from asthma worldwide, and that it was especially common in the young and the old (2017). Asthma is pulmonary hyperresponsive-ness, a type I allergy, and the symptoms vary widely, from apnea todeath (National Asthma Education and Prevention Program,N.A.E.a.P.,2002; Kay 2001). It is impossible to completely remedy asthma, and it isa major health problem (Slejko et al., 2014). There are many allergens Abbreviations: Camellia japonica, C. japonica; Th, helper T; RT-PCR, real time polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; IL, inter-leukin; WHO, World Health Organization; IFN-y, interferon gamma; TNF-a, tumor necrosis factor-alpha IgE, immunoglobulin E;OVA, ovalbumin; DEX, dex-amethasone; BALF, bronchoalveolar fluid; H&E, hematoxylin and eosin; PAS, periodic acid-schiff; PBS, phosphate-buffered saline; BM, basement membrane; cDNA,complementary deoxyribonucleic acid; RNA, ribonucleic acid; IF, immunofluorescence; IHC, immunohistochemistry; HPLC, high-performance liquid chromato-graphy; SD, standard deviation; WBC, white blood cells *Corresponding authors. E-mail addresses: sscho@mokpo.ac.kr (S.-S. Cho), dhj1221@hanmail.net (D.-H. Park). These authors equally contributed in this work. ( ht t p s :/ /doi.or g / 1 0 .1016/j.phymed.2 0 18.12.00 4 ) ( Received 5 J uly 2018;R e ceived in r e vised form 3 November 20 1 8; Ac c epted 9 December 2018 ) ( 0944-7113/ C 2018 The Au t hor(s). Published by Elsevier GmbH. This is an open a c cess article under the CC BY-NC-ND l i cense ) B D Fig. 1. Camellia japonica oil effectively controlled asthmatic factors such as inflammatory cells, eosinophil and IgE both in bronchioalveolar lavage fluid (BALF) andserum. (A) C. japonica oil significantly suppressed the number of inflammatory cells which were increased by ovalbumin treatment in BALF. (B) C. japonica oil dose-dependently downregulated the levels of IgE in serum. (C) Although there was no statistical significance C. japonica oil modulated the number of WBC in BALF. (D)The change of eosinophil number in asthma is very important and C. japonica oil significantly decreased the number of eosinophil in BALF. The level of eosinophil in100 mg/kg C. japonica oil treatment group was similar to that in dexamethasone treatment group. a, vehicle control; b, asthma induction; c, dexamethasone; d,10 mg/kg/day C. japonica oil; e, 100 mg/kg/day C. japonica oil; f, 500 mg/kg/day C. japonica oil. Each bar represents the mean ± SEM(n=8). *p <0.05 vs.control;**p <0.001 vs. control; $p <0.05 vs. asthma induction; $$p <0.01 vs.. ## asthma induction;#p <0.05 vs. dexamethasone;##p <0.01vs. dexamethasone. ScaleBar = 50 um. Magnification, x400. in the environment that can be classified into two categories: indoorallergens such as domestic mites, pet dander, etc., and outdoor allergenssuch as air pollution, chemicals, tobacco smoke, etc.(Plattes-Mills et al.,1997; Burge and Rogers, 2000). The repetitive uptake of allergens can disturb the immune system,especially Th type 1 (Th1)/Th2 system, and induce asthma occurrence(National Asthma Education and Prevention Program, N.A.E.a.P., 2002;Kay 2001). Interferon gamma (IFN-y) and interleukin 12 (IL-12) belongto Th1-related cytokine, Th2 cells release some cytokine such as IL-4,IL-5, IL-13, etc. and Th17 cell are very important cells for the mod-ulation of Th1/Th2 balance and to the onset of asthma (Mosmann andCoffman, 1989; Singh et al., 2014). In most asthma patients, the levelsof Th2-related cytokine such as IL-4, IL-5, and IL-13 are very high(Antczak et al., 2016; Foster et al., 1996; van der Pouw Kraan et al.,1998; Wenzel et al., 2007) and Th2-related cytokine is regulated byGATA-3, Th2 cell transcription factor (Yagi et al., 2011). Th1-relatedcytokines such as IFN-y and IL-12p40 downregulate Thl-related cyto-kine levels, and T-bet is a Th1 cell transcription factor (Szabo et al.,2000; Zhu et al., 2012). In order to treat asthma, inhaled combination therapy is used thesedays, and usually consists of corticosteroids and long-acting B2-agonistor leukotriene modifiers (Mishra et al., 2013). In asthma treatment, themost difficult things are that it is hard to perfectly cure, and that gen-eral medications have a lot of adverse effects such as growth suppres-sion, eye problem, hypertension, hyperlipidemia, gastric ulcer, neu-toxicity, and so on (Wise 2014; Ciriaco et al., 2013). Efforts towarddeveloping safe and effective anti-asthmatic drugs from natural products have increased in recent years (Bang et al., 2015; Lee et al.,2017; Seo et al., 2016). Camellia japonica (C. japonica) is from the Theaceae family, and re-cently, biological functions of it have been reported, such as an anti-oxidative effect andi aanti-migratory effect in breast cancer cells(Woo et al., 2017), anti-inflammatory and anti-ulcerative effect(Akanda and Park, 2017), blood pressure-lowering effect (Lim et al.,2017), anti-inflammatory and anti-oxidative effects on corneal epithe-lial cells (Lee et al., 2017), and so on. However, there is no report on thepotential anti-asthmatic effects of C. japonica oil, and in the presentstudy we investigated the anti-asthmatic effects and ingredients. Materials and methods Plant materials C. japonica seeds were collected from the Wando Arboretum(Wando, Republic of Korea) and identified by Dr. Deuk-Sil Oh affiliatedto the Wando Arboretum. Oil from C. japonica was provided by Dr.Deuk-Sil Oh and was used for further constituent analysis and in vivoexperimentation. Chemical profiling by GC-MS analysis GC-MS analysis was conducted using a previously-reported methodwith slight modifications (Bae et al., 2014). Briefly, Agilent 7890 gaschromatograph system was used to analyze scanned organic compound. Fig. 2. Camellia japonica oil dose-dependently inhibited typical asthmatic changes in the lung. (A) The photos were obtained using with hematoxylin and eosin stain,ovalbumin treatment induced the histopathological changes such mucous hyper-secretion, eosinophil infiltration, brochioalveolar epithelial cell hyperplasia, andgoblet cell hyperplasia. C. japonica oil dramatically controlled ovalbumin-induced morphological changes in the lung in a dose-dependent manner. (B) In order toevaluate the change of mouse level in the bronchioalveolar space periodic acid-schiff (PA) staining was performed. Ovalbumin stimulated the hyper-secretion ofmucous compared to both that in the control and that in the dexamethasone. However, C. japonica oil significantly inhibited the mucous secretion in a dose-dependent manner. a, vehicle control; b, asthma induction; c, dexamethasone; d, 10 mg/kg/day C. japonica oil; e, 100 mg/kg/day C. japonica oil; f, 500 mg/kg/day C.japonica oil. Scare Bar=100 um. Magnification, × 200. It was coupled to a quadrupole Agilent 5975C electron ionization(70 eV) mass spectrometric detector (Agilent Technologies, Palo Alto,CA, USA) equipped with an Agilent HP-5MS fused silica capillarycolumn (30 mm l. × 0.25 mm i.d., 0.25-um film thickness). GC-MS wastuned using perfluorotribuylamine (PFTBA) with mass fragments of69.0, 219.0, and 502.0 m/z under electron ionization (EI) conditions.The GC oven was heated as follows: isothermal at 65°C for 10 min and10 min-to 300 with helium (He) as carrier gas. Transfer line washeated at 300°C. The mass spectrometer was operated in a scan mode Animal experiments The animal study herein was conducted in the same way as ourkiaprevious study (Lee et al., 2017) and according to Kianmehr et al.(2017) the asthma occurrence was evaluated. In order to evaluate theanti-asthmatic effects of C. japonica oil and oleic acid which were Table 1 The quantitative score chart of histopathological changes in the lung. Mucous hypersecretion (0-3) Epithelial cell hyperplasia (0-3) Inflammatory cell infiltration (0-3) CON 0.1±0.35 0.4±0.52 0.1±0.35 OVA 2.9±0.35** 2.8±0.46** 2.9±0.35** DEX 0.3±0.46$ 0.6±0.52 0.6±0.52*,$ Camellia japonica 10 mg/kg 2.8±0.46**,## 2.9±0.35**,## 2.6±0.52**,## Camellia japonica 100 mg/kg 1.5±0.76**$$,## 2.4±0.52**米## 1.9±0.83**$,# Camellia japonica 500 mg/kg 0.4±0.52 0.4±0.52$$ 0.52±0.53 Each score explains the means ± standard deviation (N =8). * p<0.05 vs. control. isolated from C. japonica oil, a separate animal study was conducted.Two individual animal studies were consecutively conducted afterconfirmative study following animal study. For evaluation, the animalstudy of the anti-asthmatic effect of C. japonica oil and the confirmativestudy were done in succession. For each study, forty-eight femaleBALB/c mice were purchased from Samtako Korea (Osan, Korea) anddivided into six groups according to treatment: (1) vehicle control(sterilized tap water), (2) ovalbumin (OVA)-induced asthma model, (3)l mg/kg/day dexamethasone with ovalbumin treatment, (4)10 mg/kg/;/day C. japonica oil with ovalbumin treatment, (5) 100 mg/kg/day C.japonica oil with ovalbumin treatment, and (6) 500 mg/kg/day C. ja-ponica oil with ovalbumin treatment. On days 1 and 8, all mice exceptfor those used as the vehicle control were sensitized via intraperitonealinjections of 20 pg OVA (Sigma-Aldrich, St. Louis, MO, USA) and 1 mgaluminum hydroxide hydrate (Sigma-Aldrich) in 500 pl saline. Fromday 21 to day 25, the mice were challenged once daily with 5% OVA for30 min using a nebulizer (3ml/min, NE-U17, OMRON Co. Ltd., Kyoto,Japan). Over the same five-day period, the treatment groups were alsotreated once daily with oral doses of sterilized tap water, dex-amethasone, 10 mg/kg C. japonica oil, (5) 100 mg/kg/day C. japonicaoil, and (6) 500 mg/kg/day C. japonica oil 1 h prior to the OVA chal-lenge. The mice in the vehicle control group were sensitized with OVAaccording to the same procedures as the other groups of mice (20 ugOVA and 1 mg aluminum hydroxide hydrate in 500 ul saline), afterwhich they were exposed to saline and aluminum hydroxide hydrate bya nebulizer for five consecutive days. To evaluate the anti-asthmaticeffect of oleic acid which is the major component in C. japonica oil, andwhich was supposed as the element for ameliorating ovalbumin-in-duced asthmatic symptoms in a murine model, a very similar animalstudy was done. From this study, we confirmed the level of oleic acidwas 52.89% in C. japonica oil and based on the anti-asthmatic results ofC. japonica oil 250 mg/kg/day oleic acid treatment was decided. Theanimals were divided into four groups according to treatment: (1) ve-hicle control, (2) OVA-induced asthma model, (3) 1 mg/kg/day dex-amethasone with ovalbumin treatment, and (4) 250 mg/kg/day oleicacid with ovalbumin treatment. The last procedure was also conductedin the same as the animal study for C. japonica oil. Ethics statement All experiments were approved by the Institutional Animal Care andUse Committee atJecnnam National University (Animal StudyApproval No. CNU IACUC-YB-2018-38). BALF and serum analysis In the same way as in our previous study, BALF and serum analysiswere conducted (Lee et al., 2017; Bang et al.,2015). One day after the final treatment, the mice were anesthetized with intraperitoneal injec-tions of 50 mg/kg Zoletin (Virbac, Fort Worth, TX, USA), and theirtracheas were cannulated with disposable animal feeding needles. La-vages were performed with three 0.4 ml aliquots of cold phosphate-buffered saline (PBS). The BALF samples were collected and im-mediately centrifuged at 3000 rpm for 5 min at 4℃ (Sorvall LegendMicro 17R, Thermo Fisher Scientific, Inc.Marietta, OH, USA). The cellpellets were resuspended in PBS for total and differential cell counts.The number of total cells and differential cells were counted by theHemavet Multispecies Hematology System (Drew Scientific Inc, Wa-terbury, CT, USA). Some resuspended cell pellets were stained by theDiff-Quick method (Skipper and DeStephano, 1989). The number oftotal cells was counted using a hemocytometer, and the number ofeosinophils in BALF was counted on cytospin preparations that werestained using the Kwik-Diff staining set (Thermo Fisher Scientific Inc.,Pittsburgh, PA, USA). The levels of IgE in the serum were measured using a specific mouseIgE enzyme-linked immunosorbent assay kit (BD bioscience, 555248,San Jose, CA, USA) according to the manufacturer's protocols. Histopathological analysis Histopathological analysis was conducted as in our previous study(Lee et al.,2017). Lung tissues were fixed in 10% (v/v) formaldehydesolution, dehydrated in a graded ethanol series (99.9%, 90%, 80%, and70%), and embedded in paraffin. Paraffin-embedded lung tissues werethen sectioned (4 um) longitudinally and stained with hematoxylin andeosin. The sections were also stained with periodic acid schiff (PAS) forthe semi-quantitative analysis of glycoproteins. The images were ob-tained using an Axioscope A1 (Carl Zeiss, Gottingen, Germany). In order to clear the difference of histopathological changes amongthe groups we evaluated from 0 (none) to 3 (severe) on mucous hy-persecretion, epithelial cell hyperplasia, and inflammatory cell in-filtration. Reverse transverse-poly chain reaction (RT-PCR) analysis Fig. 3. C. japonica oil suppressed not only the expression of Th1 cell transcription factor, T-bet but also that of Th2 cell transcription factor, GATA-3. (A) The bluespots were nuclei in the cells for counter-staining. C. japonica oil perfectly inhibited both (B) the activation of helper 1 T cell transcription factor, T-bet and (C) that ofhelper 2 T cell transcription factor, GATA-3.(D) The photos meant the merge of nucleus (blue spots), T-bet (green spots), and GATA-3 (red spots). a, vehicle control;b, asthma induction; c, dexamethasone; d, 10mg/kg/day C. japonica oil; e, 100 mg/kg/day C. japonica oil; f, 500 mg/kg/day C. japonica oil. Scare Bar = 50 um.Magnification, × 1000. Immunofluorescent analysis 5'-GAAGCCCTACAGACGAGCTCA-3'; IL-5 forward 5'-TGCATCAGGGTCTCAAGTATTC-3', IL-5 reverse 5'-GGATGCTAAGGTTGGGTATGT-3';IL-13 forward 5'-CAGCCCTCAGCCATGAAATA-3', IL-13 reverse 5'-CTTGAGTGTGTAACAGGCCATTCT-3'; IL-6 forward 5'-GATAAGCTGGAGTCACAGAAGG-3',IL-6 reverse 5'-TTGCCGAGTAGATCTCAAAGTG-3;TNF-a forward 5'-CTGAGTTCTGCAAAGGGAGAG-3', TNF-a reverse5'-CCTCAGGGAAGAATCTGGAAAG-3'; GAPDH forward 5'-GTGGAGTCATACTGAACATGTAG-3', and GAPDH reverse 5'-AATGGTGAAGGTCGGTGTG-3'. The RT-PCR cycles consisted of denaturation at 95°C for 5 sand annealing/extension at 65°℃ for 30 s for 40 cycles. The results wereobtained using an Axioscope A1 (Carl Zeiss, Gottingen, Germany). In order to find the localizations of Th1 cell transcription factor, T-bet, and Th2 cell transcription factor, GATA-3 immunofluorescentanalysis was conducted, and only four groups were measured: control,OVA, dexamethasone with ovalbumin treatment, and 1500 mg/kgmacmoondong-tang with ovalbumin treatment. Prior to the antibodybinding step, the same materials for immunohistochemical analysis butrabbit anti-mouse T-bet (Biorbyt, orb7075, Cambridge, UK) or goatanti-mouse GATA-3 (OriGene, TA305795, Rockville,MD, USA) wereused as primary antibodies for 1 h at room temperature. The slides were Fig. 4. C. japonica oil dose-dependently down-regulated the expression of IL-12p40 protein. (A) C. japonica oil little affected the genes’ changes of Th1-related:.cytokine such as IFN-y and IL-12p40. However, C. japonica oil modulated the protein levels of same cytokine. (B) In the quantitative point of view, there was nostatistical significance in the change of IFN-y but (C) a slight difference was shown among C. japonica oil-treated groups in the immunohistochemical staining. (B)Compared to the change of IFN-y C. japonica oil dose-dependently suppressed the level of IL-12p40 expression and (D) the qualitative change of IL-12p40 was same tothe quantitative change of that. a, vehicle control; b, asthma induction; c, dexamethasone; d,10 mg/kg/day C. japonica oil; e, 100 mg/kg/day C. japonica oil; f,500 mg/kg/day C. japonica oil. Each bar represents the mean ± SEM (n=8). *p<0.05 vs. control; **p < 0.001 vs. control; p <0.05 vs. asthma induction;$$p <0.01 vs.asthma induction; #p <0.05 vs. dexamethasone; ##p <0.01 vs. dexamethasone. Scale Bar = 100 um. Magnification, × 200. incubated for 2h with FITC-conjugated anti-rabbit IgG (JacksonImmunoresearch,315-095-003, West Grove, PA, USA) or Alexa Flur555-conjugated anti-goat IgG (ThermoFisher Scientific, A-21127,Waltham, MA, USA) and the cells were counterstained with DAPI(ThermoFisher Scientific, 62249,Waltham, MA, USA). The images wereobtained using a K1-Fluo confocal microscope (Nanoscope System,Daejeon, South Korea). Enzyme-linked immunoassay (ELISA) analysis To analyze the levels of IFN-y, IL-12p40, IL-4, IL-5, IL-6, and TNF-ain lung tissue, OptEIA mouse ELISAs were purchased from BDBiosciences. The IL-13 levels were assessed using the AbFrontier Cymaxmouse ELISA kit (AbFrontier, Seoul, Korea). All assays were conductedaccording to the manufacturers’guidelines. All lung samples wereprepared by a lysis buffer made with a protease inhibitor cocktail andan RIPA buffer (Thermo Fisher Scientific). Aliquots of lung tissue fromall groups were weighed and homogenized with lysis buffer. They werethen centrifuged at 8200 rpm for 15 min, and the supernatants wereharvested and measured using a microplate reader (EZ Read 400,Biochrom, Cambourne,UK). Immunohistochemical analysis Immunohistochemical analysis was conducted like in our previousstudy (Lee et al., 2017). Deparaffinized tissue sections were treatedwith 3% hydrogen peroxide in methanol for 10 min in order to removeendogenous peroxidase. Antigen retrieval was carried out with sodiumcitrate buffer (0.1 M) using the hot plate method. The slides were in-cubated with normal serum in order to block nonspecific binding, thenincubated for 1h at 4℃ with primary antibodies (diluted 1:100 to 1:200) such as rat anti-mouse IFN-y monoclonal (Santa Cruz, sc-74104),rat anti-mouse IL-12p40 monoclonal (Santa Cruz, sc-57258), rat anti-mouse IL-4 monoclonal (Santa Cruz, sc-73318), rabbit anti-mouse IL-5polyclonal (Santa Cruz, sc-7887), goat anti-mouse IL-13 polyclonal(Santa Cruz, sc-1776), goat anti-mouse IL-6 polyclonal (Santa Cruz, sc-1265), and rabbit anti-mouse TNF-a polyclonal (BioVision, 3053R-100,Milpitas, CA, USA). The slides were incubated with biotinylated pan-specific secondary antibody for 10 min and then reacted with strepta-vidin peroxidase complex for 5 min (Vector Laboratories UniversalQuick Kit, Burlingame, Canada). Signals were detected using the 3,3-diaminobenzidine tetrahydrochloride substrate chromogen solution,and the cells were counterstained with Mayer's hematoxylin. The cellswere finally imaged using an Axioscope A1 (Carl Zeiss). Statistical analysis Results are expressed as mean ± standard deviation (SD). Groupdifferences were evaluated by one-way analysis of variance,followedby Dunnett's multiple comparison test. Significance was considered atp<0.01 or p<0.05. Results C. japonica oil significantly suppressed inflammatory cells and IgE level The inflammatory cells were increased more by ovalbumin treat-ment (Fig. 1.A.b,×400) than those in the control group in BALF(Fig. 1.A.a, x 400). C. japonica oil dose-dependently decreased thenumber of inflammatory cells that were up-regulated by ovalbumintreatment (Fig. 1.A.d.-A.f,× 400). In the same pattern as that in thechange of inflammatory cells, C. japonica oil effectively suppressed the 100 mg/kg E Fig.5. C. japonica oil dramatically inhibited not only cDNA levels but also protein expressions in all Th2-related cytokine such as IL-4, IL-5, and IL-13. (A) C. japonicaoil significantly and dose-dependently suppressed the genes'levels of Th2-related cytokine such as IL-4, IL-5 and IL-13 and especially the IL-5 level decreased from in100 mg/kg C. japonica oil treatment. C. japonica oil using with 100 mg/kg treatment perfectly controlled Th2-related cytokine such as IL-4, IL-5, and IL-13, not only(B) in the quantitative point of view, but also (C-E) in the qualitative point of view. a, vehicle control;b, asthma induction;c, dexamethasone; d, 10 mg/kg/day C.japonica oil; e, 100 mg/kg/day C. japonica oil; f, 500 mg/kg/day C. japonica oil. Each bar represents the mean ± SEM (n=8). *p <0.05 vs. control; **p <0.001 vs.control; $p <0.05 vs. asthma induction; $p <0.01 vs. asthma induction; #p < 0.05 vs. dexamethasone. Scale Bar=100 um. Magnification, x200. level of IgE in serum (Fig. 1.B., p <0.001 to p<0.05). The surge ofeosinphil in asthma patients is one of the important indices (Uhm et al.,2012) and the number and the levels of WBC and eosinophil in BALFwere also measured (Figs. 1.C. and D., p<0.05). The increments ofWBC and eosinophil were observed in the ovalbumin treatment groupbut dexamethasone effectively controlled the levels of WBC and eosi-nophil (p <0.001 to p<0.05). In particular, 100 mg/kg and 500 mg/kg C. japonica oil treatment significantly suppressed the level of eosi-nophil like that in the dexamenthasone treatment group (p<0.05). C. japonica oil effectively prevented the asthma typical changes inpulmonary system morphology which are caused by ovalbumin In order to evaluate the blocking effect against ovalbumin-inducedmorphological changes, H&E staining was conducted (Fig. 2.A., x 200,and Table 1.). In the ovalbumin treatment group, asthma typicalchanges in the pulmonary system were observed as mucous hyperse-cretion in the bronchioalveolar region, epithelial cell hyperplasia, in-flammatory cells infiltration near the bronchoalverolar duct and vessel,and so on (Fig.2.A.b, x200) compared to that in the control group(Fig. 2.A.a,×200). Dexamethasone which recently is used as anti- asthmaticCdrug effectively controlled ththleepulmonary changes(Fig. 2.A.c,×200) and C. japonica oil dose-dependently prevented theovalbumin-induced morphological changes (Fig. 2A.c-A.f, ×200). Inasthma patients, one of the most severe symptoms is respiratory diffi-culty, which is caused by the narrowness of bronchioalveolar ducts andthe major reason for that is mucous hypersecretion. In order to evaluatethe level of mucous in the bronchioalveloar region, PA staining wasconducted. C. japonica oil dose-dependently suppressed mucous secre-tion (Fig. 2.B.d-B.f, × 200) and especially in 500 mg/kg C. japonica oiltreatment group (Fig. 2.B.f, ×200) the level of mucous was similar tothat in dexamethasone treatment group (Fig. 2.B.e,×200). C. japonica oil controlled both the activations of Th1 cell transcriptionfactor, T-bet and Th2 cell transcription factor, GATA-3 T-bet is Th1 cell transcription factor and is modulated by IFN-y withpositive feedback (Zhu et al., 2012). Th2 cell transcription factor,GATA-3 is regulated by IL-4 with positive feedback (Yagi et al., 2011).In order to evaluate the relation of Th1-/Th2-related factors' changesthe translocation of T-bet and GATA-3 using with immunofluorecentassay was measured (Fig. 3.,×1000). In the control group, T-bet and Fig. 6. Camellia japonica oil reduced the level of Th17-related cytokine such as TNF-a and IL-6. (A) C. japonica oil reduced the relative expression gene level of Th17-related cytokines such as TNF-a and IL-6 and especially dose-dependently suppressed the gene level of TNF-a. (B) C. japonica oil controlled the levels of TNF-a and IL-6 in a dose-dependent manner. (C) According to the C. japonica oil treatment the change of TNF-a expression in the lung was shown. (D) Based on the im-munohistochemical study the change of expression level of IL-6 was similar to the that of ELISA method about IL-6. a, vehicle control; b, asthma induction; c,dexamethasone; d, 10 mg/kg/day C. japonica oil; e, 100 mg/kg/day C. japonica oil; f, 500 mg/kg/day C. japonica oil. Each bar represents the mean ± SEM (n = 8).*p <0.05 vs. control; **p <0.001 vs. control; *p < 0.05 vs.asthma induction; #p <0.05 vs.dexamethasone. Scale Bar= 100 um. Magnification, x 200. GATA-3 were located in the cyptoplasms (Fig. 3.D.a, ×1000), but inthe ovalbumin treatment group they were in the nucleus, ready to act astranscription factors (Fig. 3.D.b, ×1000). Therefore, ovalbumin sti-mulated the translocation of T-bet and GATA-3 from cytoplasm to nu-cleus for their activation. As shown in Fig. 3.D.d, C. japonica oil in-hibited the translocation of T-bet and GATA-3 from cytoplasm tonucleus like the result in the dexamethasone treatment group(Fig. 3.D.c,× 1000). C. japonica oil dose-dependently suppressed the expression ofIL-12p40 inthe lung In order to analyze the modulation effect on Th1-related cytokine byC. japonica oil, we evaluated the gene and protein levels of IFN-y and IL-12 (Fig. 4.). The expressions of gene and protein of IFN-y were noteffectively suppressed by C. japonica oil treatment (Fig. 4.A.-C., × 200,p <0.001 to p <0.05). However, the protein level of IL-12p40 wasdose-dependently controlled by C. japonica oil (Fig. 4.B. and D, ×200,p <0.001 to p<0.05), but its gene level was not (Fig. 4.A.). C. japonica oil dramatically down-regulated the levels of Th2-relatedcytokine such as IL-4, IL-5, and IL-13 There have been many reports that Th2-related cytokine plays animportant role in various hyper-responsiveness such as asthma (Busse,2001; Wills-Karp, 1999). In most asthma patients, the levels of Th2-related cytokines such as IL-4, IL-5, and IL-13 are highly elevated fromnormal functioning. In order to measure the changes in Th2-relatedcytokines levels by C. japonica oil treatment, both gene level and pro-tein level were analyzed (Fig.5.). The IL-4 gene level significantly in-creased by ovalbumin treatment but C. japonica oil effectively and dose-dependently suppressed its level (Fig. 5.A., p <0.001 to p<0.05). Similar to the result of gene level C. japonica oil significantly controlledthe protein expression of IL-4 which increased by ovalbumin treatment(Fig. 5.B. and C., x 200, p<0.001 to p<0.05). C. japonica oil sup-pressed not only the levels of gene and protein of IL-5 (Fig. 5.B. andD.,×200,p <0.001 to p <0.05) but also those of IL-13 (Fig. 5.B. andE.,x200, p<0.001 to p<0.05). In particular, 100 mg/kg and500 mg/kg C. japonica oil treatment inhibited all of the protein ex-pressions of Th2-related cytokines (Fig. 5.B., p <0.001 to p <0.05). C. japonica oil dose-dependently suppressed the protein level of Th17-relatedcytokine, TNF-a Th17 cells may regulate Th1 cells and Th2 cells, and then play animportant role in hyperresponsiveness such as in the case of asthma(Zhao et al., 2013). We evaluated the gene and protein levels of Th17-related cytokines such as TNF-a and IL-6 (Fig. 6.). Although C. japonicaoil did not effectively suppress the gene and protein levels of IL-6 itcould control those of TNF-a with statistical significance (Fig. 6.A. andB., p <0.001 to p<0.05). As shown in Fig. 6.D. (×200), C. japonicaoil significantly inhibited the expression of TNF-a, which was upregu-lated by ovalbumin treatment (p <0.001 to p < 0.05). Identification of active constituents in C. japonica Typical GC-MS chromatograms of phytochemical contents and theirretention times are shown in Fig. 7. Major compounds [i.e., oleic acid(52.89%, Fig. 7.A.), 6-octadecenoic acid (9.12%,Fig. 7.B.), 9,12-octa-decadienoic acid (3.32%, Fig. 7.C.), 1-mono-oleoylglycerol (2.68%,Fig. 7.D.), B-amyrin (2.15%, Fig. 7.E.), and octadecanoic acid (2.13%,Fig. 7.F.)] were identified by GC-MS analysis. As was shown in Fig. 7.G.we got the result of total ion chromatogram of C. japonica. A oleic acidR.T.:28.85 min B 6-octadecenoic acid R.T.:28.18 min octadecanoic acid D 1-mono-oleoylglycerolR.T. : 33.08 min E B-amyrinR.T.: 39.36 min G Total Ion Chromatogram (TIC) R.T.(min.) 30 50 Fig. 7. GC chromatogram of Camellia japonica oil (A) Octadecanoic acid, (B) 1-monooleoylglycerol,(C) b-Amyrin, (D) 6-Octadecenoic acid, (E) Oleic acid, (F) 9,12-Octadecadienoic acid, (G) Total Ion Chromatogram (TIC). Oleic acid was the major component in C. japonica oil and significantlysuppressed typical asthmatic changes Discussion In order to evaluate the anti-asthmatic effect of oleic acid, which isthe major component in C. japonica oil using with asthma murinemodel, we additionally conducted the animal experiment. We alsoevaluate the typical asthmatic changes such as inflammatory cell po-pulation, WBC, and eosinophil in BALF, IgE level in serum and mor-phological changes in the lung (Fig. 8.). As the level of oleic acid was52.89% in C. japonica oil 250 mg/kg, oleic acid treatment, which mightbe similar to the maximum dose of C. japonica oil, of 500 mg/kg wasused for this study. The inflammatory cell proliferation which wascaused by ovalbumin treatment (Fig. 8.A.b, × 400) wa decreased byoleic acid treatment (Fig. 8.A.d,×400). The results of WBC and eosi-nophil were very similar to those of inflammatory cells in BALF(Fig. 8.B., p<0.05). Oleic acid significantly suppressed the level of IgEin the serum (Fig. 8.C., p <0.001 to p <0.05). In order to evaluate thepulmonary morphological changes, H&E staining and PAS stainingwere conducted (Fig. 8.D. and E., × 200). Oleic acid effectively pre-vented the asthma typical changes such as mucous secretion, in-flammatory infiltration, epithelial cell hyperplasia, and so on. In par-ticular, the suppression level of asthma treated by oleic acid occurrencewas similar to that by dexamethasone. Immunological tissue damage is classified as one of four categories(Todd et al.,2015). Type I is immediate hypersensitivity and is medi-ated with IgE. Type II relates with cell or membrane reaction andmediates with IgG, IgA, or IgM. Type III and type IV mediate immunecomplex and cell, respectively. Asthma is involved in Type I im-munological tissue damage and mediated with IgE (Ariza et al., 2014).Asthma stimulators, which are called allergens, are categorized intotwo groups: indoor allergens such as house-dust-mite, pet dander,cockroach, etc. (Plattes-Mills et al., 1997), and outdoor allergens suchas pollen, soy dust, fungi, arthropods, etc. (Burge and Rogers, 2000).These days in particular, fine dust might be one of the outdoor allergensbehind asthma occurrence (Yonhap New Agency, 2017). The symptoms in ovalbumin murine asthma model are very similarto those in asthma patients and the pathogenic mechanism was sup-posed to have an imbalance relationship between the Th1 cell and Th2cell (Lee, et al., 2017). Th1 cells could produce many cytokines such asIL-12, IFN-y, etc., and Th2 cells could produce several cytokines such asIL-4, IL-5, IL-13, and so on (Mosmann and Coffman, 1989). Th1 celltranscription factor is T-bet, which could make IFN-y with positivefeedback (Szabo et al., 2000; Zhu et al., 2012) and GATA-3, Th2 celltranscription factor is controlled by IL-4 (Yagi et al., 2011). IL-12p40 isone of the important factors that can control Th1-related cytokine and D E Fig.8. Oleic acid had anti-asthmatic effect in ovalbumin-induced asthma animals as similar to the results of Camellia japonica oil. (A) Oleic acid which was isolatedfrom C. japonica oil significantly inhibited the level of inflammatory cells in BALF. Magnification, × 400 (B) The decreased levels of WBC and eosinophil by oleic acidwere similar to the changes of them by dexamethasone. (C) Oleic acid inhibited the level of IgE which was induced by ovalbumin as similar to that by dexamethasone.(D) Oleic acid controlled the typical asthmatic change of lung such as mucous hyper-secretion, bronchioalveolar epithelial layer hyperplasia, inflammatory cellsinfiltration near the bronchoialveola and vessel, and so on. Magnification,× 200. (E) Oleic acid decreased the mucous secretion similar to the level of that bydexamethasone. Magnification, × 200; a, vehicle control; b, asthma induction; c, dexamethasone; d, 10 mg/kg/day C. japonica oil; e, 100 mg/kg/day C. japonica oil;f, 500 mg/kg/day C. japonica oil. Each bar represents the mean ± SEM (n=8). *p<0.05 vs. control; **p <0.001 vs. control; $p <0.05 vs. asthma induction;sp<0.01 vs. asthma induction. Scale Bar =100 um. induce IFN-y releasing and is related to asthma (Hamaza et al., 2010).C.japonica oil more effectively modulated the change of IL-12p40 thanIFN-y (Fig. 4.B.). There have been many reports that in asthma patients,Th2-related cytokines, such as IL-4, IL-5, IL-13, etc.,were much higherthan those in healthy people (Antczak et al., 2016; Foster et al., 1996;van der Pouw Kraan et al., 1998; Wenzel et al., 2007). IL-4 plays animportant role in asthma, as induces the production of eoshinophil andneutrophil via mast cell stimulation, produces Th2-related cytokine viaGATA-3 activation, stimulates IgE production via B cell activation, ex-cessively secretes mucous, and so on (Larche et al., 2003; Lee and In2006). IL-5 stimulates the survival and proliferation of eosinophil(Kouro and Takatsu 2009) and finally relates the increment of eosino-phil in BALF and eosinophil infiltration in the lung. The asthma-relatedfunctions of IL-13 are very similar to those of IL-4;it stimulates IgE,eosinophil, and basophil production, induces mucous hypersecretion(Larche et al., 2003; Platts-Mills, 2001). However, the differentialfunctions between IL-13 and IL-4 are to induce airway hyperrespon-siveness (AHR) and airway remodeling such as inflammation, fibrosis,obstruction, etc. in the lung (Zhu et al., 1999). Although C. japonica oilsuppressed both Thl-related cytokines, IL-12 and Th2-related ones, IL-4, IL-5, and IL-13, it effectively controlled much more the Th1-relatedone than the Th2-related cytokine (Figs. 4. and 5.). The gene and pro-tein levels of IL-4, IL-5, and IL-13 in 100 and 500 mg/kg C. japonica oiltreatment groups were similar to those in dexamethasone treatmentgroup only except the results of IL-13 (Figs. 5.A. and B.). Th17 cell isone of the very important factors to modulate asthma occurrence(Singh et al., 2014), chemo-attracts neutrophil andeosinophil (Lukacs et al., 1995) and stimulates eosinophil's cell death via oxidativestress in respiratory epithelial cells (Slungaard et al., 1990). C. japonicaoil effectively inhibited the TNF-a releasing (Fig. 6.A. to C.). Asthma is an incurable pulmonary disease (Slejko et al., 2014), andthe one way to control it is to relieve its symptoms. In the present,corticosteroid hormone has been broadly used for the suppression ofthat by inhalation method, but has many adverse effects: children'sgrowth inhibition, central nervous disorder, cataract and glaucoma,hyper-lipidemia, peptic ulcer, and so on (Wise, 2014; Ciriaco et al.,2013). As inhaled medication for asthma treatment has many side ef-fects, many efforts have recently been carried out to find the anti-asthmatic drug candidates which have relatively lower adverse effects. β-Amyrin, has been shown to exert anti-inflammatory effect on di-methyl nitrosamine-induced hepatic fibrosis in male rats(Thirupathi et al., 2017). Oleic acid is a naturally occurring fatty acidfound in animal fats and vegetable oils. It has several biological effectssuch as reducing low-density lipoprotein and increasing high-densitylipoprotein,reducing blood pressure (Peyrat-Maillard et al., 2003), andanti-inflammation (Erdinest et al., 2015). Although there have beenreports that a low concentration of oleic acid exacerbates LPS-inducedcell death and inflammation in human alveolar epithelial cells(Kucukgul and Erdogan, 2017), the relation of oleic acid and asthmaoccurrence or prevention remains unclear. Chung et al., (2015) re-ported that oleic acid suppressed peanut and cashew nut-related IgEincrement. In the present study we clearly elucidated that oleic acidsuppressed typical asthmatic changes such as inflammatory cell pro-liferation, the increase of WBC and eosinophil in BALF, IgE surge in serum, and morphological changes in the lung (Fig. 8). From these results, we concluded that although further study isneeded for the mode of action, the major anti-asthmatic ingredient in C.japonica oil was oleic acid, and C. japonica oil is a promising candidatefor asthma preventive protocol. Conflict of interest The authors declare no competing financial interest. Acknowledgment This research was supported by Basic Science Research Programthrough the National Research Foundation of Korea (NRF) funded bythe Ministry of Education (2015R1D1A1A01059523). References Akanda, M.R., Park, B.Y., 2017. Involvement of MAPK/NF-kB signal transduction path-ways: Camellia japonica mitigates inflammation and gastric ulcer. BiomedPharmacother 95, 1139-1146. Antczak,A., Domanska-Senderowska, D., Gorski, P., Pastuszak-Lewandoska, D.,Nielepkowicz-Gozdzinska, A., et al.,2016. Analysis of changes in expression of IL-4/IL-13/STAT6 pathway and correlation with the selected clinical parameters in pa-tients with atopic asthma. Int. J. Immunopathol. Pharmacol. 29 (2),195-204. Ariza, A., Fernandez, T.D., Dona,I., Aranda, A., Blanca-Lopez, N., Melendez, L., Canto, G.,Blanca, M., Torres, M.J., Mayorga, C., 2014. Basophil activation after nonsteroidalanti-inflammatory drugs stimulation in patients with immediate hypersensitivityreactions to these drugs. Cytometry A 85, 400-407. Bae, M.S., Shin, J.S., Lee, K.Y., Lee,K.H., Kim, Y.J., 2014.Long-range transport of biomassburning emissions based on organic molecular markers and carbonaceous thermaldistribution. Sci Total Environ 466-467, 56-66. ( B an g, M . A. , S eo, J.H . , S e o , J . W . , J o, G . H . , J u n g , S . K., Y u , R ., P a r k, D . H., Pa r k , S .J . , 2 0 1 5. B acillus s ubtili s KCTC 11 782BP-pr o duce d alg i n ate o li g os a cc hari d e e ff ec t i vely su p - p res ses a s t hma v i a T- h el p er c ell t y p e 2- r e l a ted c ytokin e s . PLoS O n e 1 0 , e 01 1 75 2 4 . ) Burge, H.A., Rogers, C.A., 2000. Outdoor allergens. Environ. Health Persp. 108 (4),653-659. ( B u s s e , W. W ., 2 0 01. A s th ma. N . E n g l. J . M ed . 3 4 4, 3 5 0- 3 6 2 . ) ( C hun g , S.Y. , M atti s on, C . P . , Reed, S . , Wa s serm a n, R. L ., 2015 . Tr eatmen t w i th o l e i c acid r e d uces Ig E bindi ng to peanut and c ash e w a lle rg e n s. Foo d Ch e m . 1 80 , 2 95- 3 00. ) Ciriaco, M., Ventrice, P., Russo, G., Scicchitano,M., Mazzitello, G., Scicchitano, F., Russo,E., 2013. Corticosteroid-related central nervous system side effects. J PharmacolPharmacother 4, S94-S98. Erdinest, N., Shohat, N., Moallem, E., Yahalom, C., Mechoulam, H., Anteby,I.,Ovadia, H.,Solomon, A., 2015. Nitric oxide secretion in human conjunctival fibroblasts is in-hibited by alpha linolenic acid. J. Inflamm. (Lond). 12, 59. Foster, P.S., Hogan, S.P.,Ramsay,A.J., Matthaei, K.I., Young, I.G., 1996. Interleukin 5deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in amouse asthma model. J. Exp. Med. 183,195-201. Hamaza, T., Barnett, J.B., Li, B., 2010. Interleukin 12 a key immunoregulatory cytokine ininfection applications. Int. J. Mol. Sci. 11,789-806. Kay, A.B., 2001. Allergy and allergic diseases. First of two parts. N. Engl.J. Med. 344,30-37. Kianmehr, M., Ghorani, V., Boskabady, M.H., 2017. Animal model of asthma, variousmethods and measured paramenters: a methodological review. Iran J. Allergy AsthmaImmunol. 15 (6), 445-465. Kouro, T., Takatsu, K., 2009. IL-5- and eosinophil-mediated inflammation: from discoveryto therapy. Int Immunol 21 (12), 1303-1309. Kucukgul, A., Erdogan, S., 2017. Low concentration of oleic acid exacerbates LPS-inducedcell death and inflammation in human alveolar epithelial cells. Exp. Lung. Res. 43(1),1-7. Larche, M., Robinson, D.S., Kay, A.B., 2003. The role of T lymphocytes in the patho-genesis of asthma. J. Allergy Clin. Immunol. 111 (3), 450-463. Lee, H.S., Choi, J.H., Cui,L., Li, Y., Yang, J.M., Yun, J.J., Jung, J.E., Choi, W., Yoon, K.C.,2017a. Anti-inflammatory and anti-oxidative effects of Camellia japonica on humancorneal epithelial cells and experimental dry eye: in vivo and in vitro study. Invest.Ophthalmol. Vis. Sci. 58 (2), 1196-1207. Lee, S.Y., Bae, C.S., Choi, Y.H.,Seo, N.S., Na,C.S., Yoo, J.C., Cho,S.S., Park, D.H., 2017b.Opuntia humifusa modulates morphological changes charateristic of asthma via IL-4and IL-13 in an asthma murine model.Food Nutr. Res. 61, 1-12. Lee, S.Y., In, K.H., 2006. Immunopathogenesis of asthma. Tubercul. Respir. Dis. 60 (4), ( 3 79- 3 90. ) Lim, H.J., Kim, M.S., Kim, D.S., Kim, Y.J., Lee, J.H., Pan, J.H.,Shin, E.C., Kim, J.K., 2017.Blood pressure-lowering effects of alacalase-hydrolyzed Camellia seed hull in vitro andin spontaneous hypertensive rat. J. Med. Food 20 (7),720-723. Lukacs, N.W., Strieter, R.M., Chensue, S.W., Widmer, M., Kunkel, S.L., 1995.TNF-alphamediates recruitment of neutrophils and eosinophils during airway inflammation. J.Immunol. 154, 5411-5417. Mishra, A., Yao, X., Levine, S.J., 2013. From bedside to bench to clinic trials: identifyingnew treatments for severe asthma. Dis Model Mech 6, 877-888. Mosmann, T.R., Coffiman, R.L., 1989. Th1 and Th2 cells: different patterns of lymphokinesecretion lead to different functional properties. Annu. Rev. Immunol. 7, 145-168. National Asthma Education and Prevention Program, N.A.E.a.P., 2002. Expert panel re-port: guidelines for the diagnosis and management of asthma update on selectedtopics-2002. J. Allergy Clin. Immunol. 110, S141-S219. Peyrat-Maillard, M.N., Cuvelier, M.E., Berset, C., 2003. Antioxidant activity of phenoliccompounds in 2,2'-azobis (2-amidinopropane) dihydrochloride (AAPH)-inducedoxidation: Synergistic and antagonistic effects. J. Am. Oil Chem. Soc. 80, 1007. Platts-Mills, T.A.E., Vervloet, D, Thomas, W.R., Aalberse, R.C., Chapman, M.D., 1997.Indoor allergen and asthma: report of the third international workshop. J. AllergeyClin. Immunol. 100 (6, part 1), S2-S24.上Platts-Mills, T.A., 2001. The role of immunoglobulin E in allergy and asthma. Am. J.Respir. Crit. Care Med. 164, S1-S5. van der Pouw Kraan, T.C., van der Zee, J.S., Moeije, L.C., de Groot, E.R., Stapel, S.O.,Aarden, L.A., 1998. The role of IL-13 in IgE synthesis by allergic asthma patients.Clin. Exp. Immunol. 111, 129-135. Seo,J.H., Bang, M.A., Kim, G., Cho, S.S., Park, D.H., 2016. Erythronium japonicum at-tenuates histopathological lung abnormalities in a mouse model of ovalbumin-in-duced asthma. Int J Mol Med 37, 1221-1228. Singh, A.,Yamamoto, M., Ruan, J., Choi, J.Y., Gauvreau, G.M., Olek, S., Hoffmueller, U.,Carlsten, C., FitzGerald,J.M., Boulet, L.P., O'Byrne, P.M., Tebbutt, S.J., 2014. Th17/Treg ratio derived using DNA methylation analysis is associated with the late phaseasthmatic response. Allergy Asthma Clin. Immunol. 10, 32. Skipper, R., DeStephano, D., 1989. Diff-Quik stain set. J. Histotechnol. 12 (4), 303. Slejko, J.F.,Ghushchyan, V.H., Sucher, B., Globe, D.R., Lin, S.L., Globe, G., Sullivan, P.W.,2014. Asthma control in the United States,2008-2010: indicators of poor asthmacontrol. J. Allergy Clin. Immunol.133,1579-1587. Slungaard, A., Vercellotti, G.M., Walker, G., Nelson, R.D., Jacob, H.S., 1990. Tumor ne-crosis factor alpha/cachectin stimulates eosinophil oxidant production and toxicitytowards human endothelium. J. Exp. Med. 171, 2025-2041. Szabo, S.J., Kim, S.T., Costa, G.L., Zhang, X., Fathman, C.G., Glimcher, L.H., 2000. Anovel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655-669. Thirupathi, A., Silveira, P.C., Nesi, R.T., Pinho, R.A., 2017. Beta-Amyrin, a pentacyclictriterpene, exhibits anti-fibrotic, anti-inflammatory, and anti-apoptotic effects ondimethyl nitrosamine-induced hepatic fibrosis in male rats. Hum. Exp. Toxicol. 36,113-122. Todd,I., Spickett, G., Fairclough, L., 2015. Mechanisms of immunological tissue damage.Immunology Lecture Notes, Seventh ed. John Wiley & Sons. Ltd., West Sussex, UK. Uhm, T.G., Kim, B.S., Chung, I.Y., 2012. Eosinophil development, regulation of eosino-phil-specific genes, and role of eosinophils in the pathogenesis of asthma. AllergyAsthma Immunol. Res. 4(2),68-79. Wenzel, S., Wilbraham, D., Fuller, R., Getz, E.B., Longphre, M., 2007. Effect of an iter-leukin-4 variant on late phase asthmatic response to allergen challenge in asthmaticpatients: results of two phase 2a studies. Lancet 370, 1422-1431. Wills-Karp, M., 1999.Immunologic basis of antigen-induced airway hyperresponsiveness.Annu. Rev. Immunol. 17, 255-281. Wise, J., 2014. Corticosteroids for asthma may suppress growth in children in first year oftreatment, researchers say. BMJ 349, g4623.9,8402 Woo, Y., Lee, H.,Jeong, Y.S., Shin, G.Y., Oh, J.G., Kim, J.S., Oh, J., 2017. Antioxidantpotential of selected Korean edible plant extracts. Biomed Res. Int. 2017, 7695605.World Health Organization, August 31 2017,. Asthma. Yagi, R., Zhu, J., Paul, W.E., 2011. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 23 (7),415-420. Yonhap New Agency, November 07, 2017. Fine Dust Overexposure Leads to Rise ofAsthma Patients: Study. Yonhap News. Zhao, J., Lloyd,C.M., Noble, A., 2013. Th17 responses in chronic allergic airway in- flammation abrogate regulatory T-cell-mediated tolerance and contribute to airwayremodeling. Mucosal Immunol 6 (2), 335-346. Zhu, J., Jankovic,D., Oler, A.J., Wei, G., Sharma, S., Hu, G., Guo, L., Yagi, R., Yamance,H., Punkosdy, G., Feigenbaum, L., Zhao, K., Paul, W.E., 2012. The transcription factorT-bet is induced by multiple pathways and prevents an endogenous T helper-2 pro--higram during T helper-1 responses. Immunity 37 (4), 660-673. Zhu, Z., Homer, R.J., Wang, Z., Chen, Q., Geba, G.P., Wang, J., Zhang, Y., Elias, J.A., 1999. Pulmonary expression of interleukin-13 causes inflammation, mucus hy-persecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin produc-tion. J Clin Invest 103, 779-788. 根据世界卫生组织的报告, 每年都有数十万的人死于哮喘病,另外大约有两亿的人正受到喘病的折磨。虽然目前市场上有很多的抗哮喘药物,但是有着很多的副作用。因此发新的抗哮喘药物迫在眉睫。哮喘病是肺部过度反应,是I型过敏,症状从呼吸暂停到死亡。在哮喘病治疗过程中最困难的是哮喘病很难被彻底治愈,而且基因药物有很多不良反应,如眼部问题,高血压,高脂血症,胃溃疡,毒性等。因此开发天然有效的抗哮喘药物就显得尤为重要。2019年有韩国Dongxin 大学Oriental医学院, Chonnam大学Veterinary医学院,Sangmyung 大学生物技术系,Jeollanam-do 森林资源研究所,Mokpo国立大学工程学院等数家科研单位共同合作研究山茶油(C. japonica oil)对于抑制哮喘病的功效。在研究的过程中,我们利用韩国Nanoscope System 公司的荧光共聚焦显微镜对细胞的免疫荧光进行了分析。在实验中为了找到Th1细胞转录因子,Tbet和Th2细胞转录因子的定位,进行了GATA-3免疫荧光分析,仅测量了四组:对照组,OVA,地塞米松和卵清蛋白处理以及1500 mg / kg的macmoondong唐用卵清蛋白治疗。在抗体结合步骤之前,使用相同的材料进行免疫组织化学分析,但使用兔抗小鼠T-bet或山羊抗小鼠GATA-3。在室温下用作一抗1小时。将载玻片与结合FITC的抗兔IgG或Alexa Flur 555结合的抗山羊IgG,并将细胞用DAPI复染。 然后使用K1-Fluo 激光荧光共聚焦显微镜对细胞进行观察,获得超高清晰度的细胞荧光图像。
关闭-
1/11

-
2/11
还剩9页未读,是否继续阅读?
继续免费阅读全文产品配置单
Nanoscope Systems,Inc.为您提供《细胞中生物检测方案 》,该方案主要用于细胞中生物检测,参考标准《暂无》,《细胞中生物检测方案 》用到的仪器有 K1-Fluo ABM 激光荧光共聚焦显微镜。
我要纠错
推荐专场
相关方案






 咨询
咨询


