方案详情文
智能文字提取功能测试中
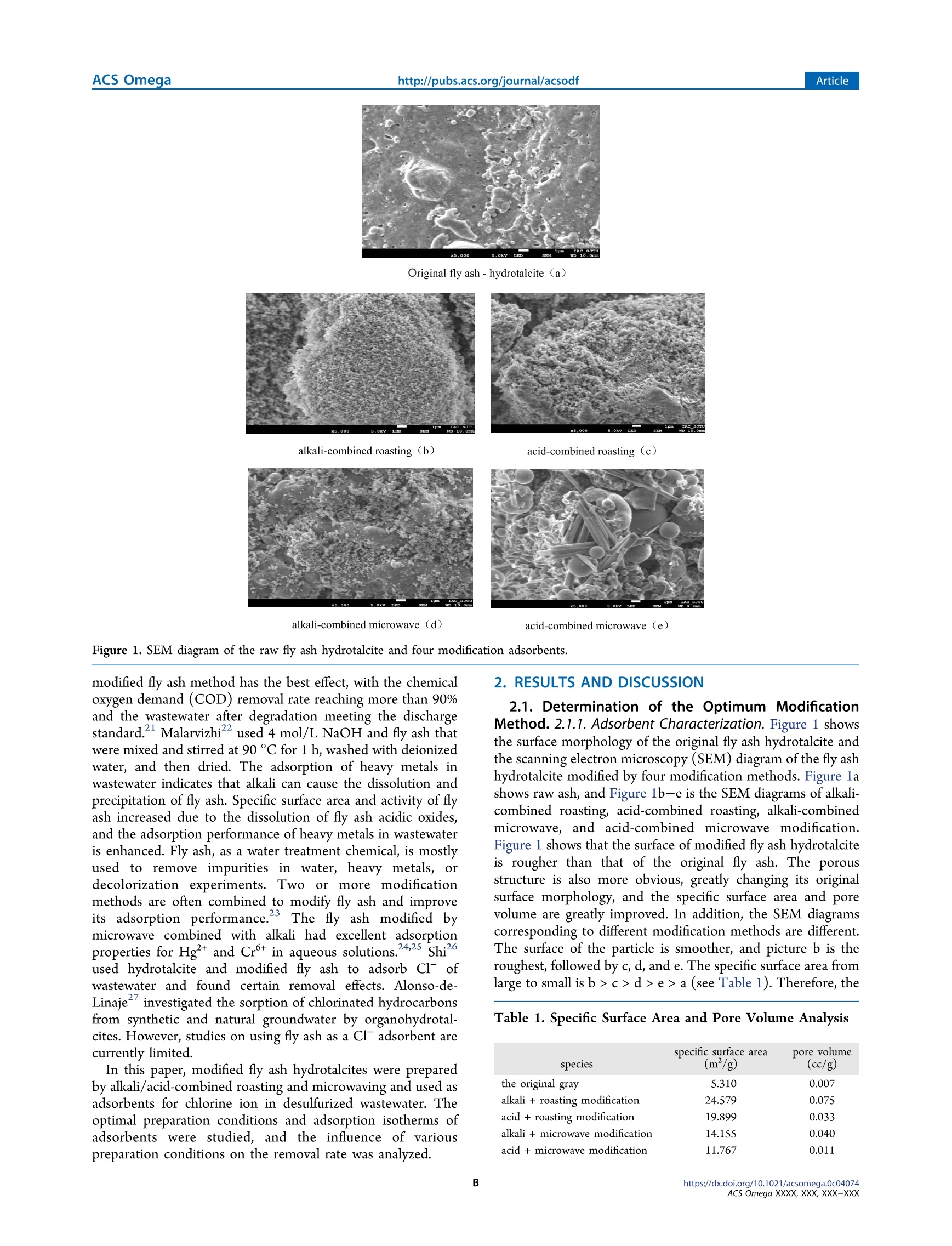
This is an open access article published under a Creative Commons Non-Commercial NoDerivative Works (CC-BY-NC-ND) Attribution License, which permits copying andredistribution of the article, and creation of adaptations, all for non-commercial purposes.AUTHORCHOICEhttp://pubs.acs.org/journal/acsodf ArticleACS Omegahttp://pubs.acs.org/journal/acsodf Article Removal of Chlorine lons from Desulfurization Wastewater byModified Fly Ash Hydrotalcite Liqiang Qi,* Kunyang Liu, Ruitao Wang, Jingxin Li,* Yajuan Zhang, and Lan Chen Cite This: https://dx.doi.org/10.1021/acsomega.0c04074 Read Online ACCESS Metrics & More Article Recommendations ABSTRACT: The effective removal of chlorine ion from thedesulfurization slurry is of great significance to the stable operationof the desulfurization system. Modified fly ash hydrotalcites wereprepared by alkali/acid-combined roasting and microwaving andused as an adsorbent for chlorine ion in desulfurized wastewater. Alkali combined The specific surface area and porosity of different adsorbents were analyzed by X-ray diffraction (XRD) and scanning electronmicroscopy (SEM). The impacts of pH, temperature, adsorbent dosage, and adsorption shaking time on adsorption performancewere investigated. Results showed the alkali-combined roasting-modified fly ash hydrotalcite has the optimum removal effect on Cl.The optimal adsorption performance was achieved when the pH was 8, the adsorption temperature was 60 C, the massconcentration of adsorbent was 10 g/L, the adsorption shaking time was 180 min, and the removal percentage of Cl was 68.1%.The adsorption isotherm was consistent with the Langmuir isotherm model, and the adsorption saturation was 694.4 mg/g, whichbelonged to monolayer adsorption. 1. INTRODUCTION Desulfurization wastewater contains a considerable amount ofsuspended solids (e.g.,gypsum particles, SiO2,aluminum, andiron hydroxide), inorganic salts, and trace heavy metals, such asarsenic, cadmium, chromium, and mercury, thus seriouslyharming the environment if directly discharged. Thedissolution of chlorides in the flue gas will increase thechloride ion concentration in the desulfurization absorbent.This increase will decrease the desulfurization rate and affectthe gypsum quality, resulting in the corrosion of desulfurizationsystems. After the triple box process of “neutralization,coagulation, and precipitation", the content of heavy metalsand suspended solids in desulfurization wastewater is reducedbut that of chlorine remains high. The 2017 guidelines onfeasible technologies for the prevention and control ofpollution in thermal power plants (HJ2301-2017) indicatedthat the key to achieving a near-zero discharge of wastewater is2-4to realize a zero discharge of desulfurization wastewater.The main methods currently used to remove Cl fromwastewater include electric coagulation, ion exchange, andadsorption.Adsorption is regarded as one of the effectiveways to uptake Cl from wastewater due to its advantages ofsimple operation, recyclability, etc. Fly ash is a byproduct of coal-fired power plants. China is aPowerplangcahlarge producer and user of coal. The coal-fired powergeneration of China can produce hundreds of millions ofns of fly ash yearly.3-10 FFllyy aasshh iiss characterized by its lowcost, easy acquisition, porous physical tissue, large specificarea, and high adsorption activity.-13 Therefore, thetreatment of harmful substances in wastewater using fly ash hasbeen widely investigated. Studies on the removal of phosphorus from wastewater by fly ash began in 1980 whenKuziemska applied the water extract of brown fly ash as acoagulant to treat phosphorus in wastewater.4 Mohan usedfly ash as a cheap adsorbent to treat heavy metal ions in urbansolid waste leachates. Studies have shown that modified fly ashhas a larger specific surface area and a stronger adsorptioncapacity than those of unmodified fly ash.16 Therefore, usingmodified fly ash to treat some harmful substances is possibledue to its strong adsorption performance. Wang used theH SO4 impregnation method to modify raw coal fly ash(RCFA) and used the modified RCFA (ACFA) as a Fenton-like catalyst to treat polymer flooding wastewater (PW) undermicrowave prestrengthening conditions. The fly ashwasmodified by nitric acid at room temperature, aand theadsorption performance of the modified fly ash to methyleneblue was significantly improved.l8 Nguyen used 2-mercapto-benzothiazole (MBT) and sodium dodosulfate (SDS)) aassurfactants and treated fly ash with sodium hydroxide toinvestigate the morphology, composition, andstructuralchanges of modified and unmodified fly ash as well as theadsorption behavior of Cd’ and Hg2. Sahoo studied theremoval of heavy metals from acidic mine wastewater by alkali-modified fly ash. Research by Li and others found that the acid- ( Rec ei v ed : A ugust 2 3, 2020 ) ( A c ce pte d: October 28, 2020 ) modified fly ash method has the best effect, with the chemicaloxygen demand (COD) removal rate reaching more than 90%and the wastewater after degradation meeting the dischargestandard.Malarvizhi used 4 mol/L NaOH and fly ash thatwere mixed and stirred at 90 °C for 1 h, washed with deionizedwater, and then dried. The adsorption of heavy metals inwastewater indicates that alkali can cause the dissolution andprecipitation of fly ash. Specific surface area and activity of flyash increased due to the dissolution of fly ash acidic oxides,and the adsorption performance of heavy metals in wastewateris enhanced. Fly ash, as a water treatment chemical, is mostlyused to remove impurities in water, heavy metals, ordecolorization experiments. Two or more modificationmethods are often combined to modify fly ash and improveits adsorption performance.23 The fly ash modified bymicrowave combined with alkali had excellent adsorptionproperties for Hg’ and Crt in aqueous solutions.24,25 Shiused hydrotalcite and modified fly ash to adsorb Cl ofwastewater and found certain removal effects. Alonso-de-Linaje investigated the sorption of chlorinated hydrocarbonsfrom synthetic and natural groundwater by organohydrotal-cites. However, studies on using fly ash as a Cl adsorbent arecurrently limited. In this paper, modified fly ash hydrotalcites were preparedby alkali/acid-combined roasting and microwaving and used asadsorbents for chlorine ion in desulfurized wastewater. Theoptimal preparation conditions and adsorption isotherms ofadsorbentss were studied, and the influence of variouspreparation conditions on the removal rate was analyzed. 2. RESULTS AND DISCUSSION 2.1. Determination of the Optimum ModificationMethod. 2.1.1. Adsorbent Characterization. Figure 1 showsthe surface morphology of the original fly ash hydrotalcite andthe scanning electron microscopy (SEM) diagram of the fly ashhydrotalcite modified by four modification methods. Figure lashows raw ash, and Figure 1b-e is the SEM diagrams of alkali-combined roasting, acid-combined roasting, alkali-combinedmicrowave, and acid-combined microwave modification.Figure 1 shows that the surface of modified fly ash hydrotalciteis rougher than that of the original fly ash. The porousstructure is also more obvious, greatly changing its originalsurface morphology, and the specific surface area and porevolume are greatly improved. In addition, the SEM diagramscorresponding to different modification methods are different.The surface of the particle is smoother, and picture b is theroughest, followed by c, d, and e. The specific surface area fromlarge to small is b>c > d> e> a (see Table 1). Therefore, the Table 1. Specific Surface Area and Pore Volume Analysis specific surface area pore volume species (m/g) (cc/g) the original gray 5.310 0.007 alkali + roasting modification 24.579 0.075 acid+roasting modification 19.899 0.033 alkali + microwave modification 14.155 0.040 acid + microwave modification 11.767 0.01 adsorption capacity is also different. The alkali-combinedroasting modification method has a more evident removalpercentage of Cl compared with that of other modificationmethods. Thus, the SEM surface of this method is rough andits porous structure is evident. Therefore, the adsorption effectof this method is better. Table 1 shows the specific surface area and pore volume offly ash and modified fly ash hydrotalcite. The specific surfacearea of the adsorbent modified by alkali roasting was 24.579m /g, which was considerably larger than that of the originalfly ash and increased by approximately fivefold. The OHdissolved from fly ash and Nat and K* in alkaline modifierdestroyed Si-O and Al-O bonds in the fly ash vitreous body,depolymerized the silicate glass network, promoted theformation of zeolite skeleton from fly ash, and increasedmany new active points in alkali modification, as shown inFigure 2; thus, the physical and chemical adsorptionperformances of fly ash are improved.28,29 Figure 2. Aircraft layout of alkali-modified fly ash. The volume and the surface area of fly ash increased duringhigh-temperature roasting and modification. This increasecould be attributed to the melting of fusible substances in flyash, the destruction of the glass network structure, thedepolymerization of the original high polymer silicate network,and the loose and porous fly ash particles. Simultaneously, thewater on the surface of fly ash particles at the beginning ofhigh-temperature roasting modification was evaporated astemperature increased; this phenomenon exposed additionaladsorption activity points on the fly ash surface and promotedthe improvement of the adsorption capacity of fly ash. 2.1.2. Removal Percentage of C厂 by Four Adsorbents.Figure 3 shows that the removal percentages of Cl using thefour modified methods were 56.9, 45.2, 39.7, and 19.2%. Theseresults showed that adsorbent A had the optimum removaleffect on Cl in the desulfurization wastewater. Strong alkalican destroy the Si-O-Si and Si-O-Al network structure indepolymerized fly ash and can act as an activator to increasethe activity of fly ash and improve the physical and chemicaladsorption properties of fly ash, The high-temperature roastingmakes the moisture inside the fly ash evaporate, and morepores are formed on the fly ash surface, which increases thesurface active site of the fly ash. Therefore, the alkali-combinedroasting-modified fly ash thydrotalcite was;used in thesubsequent optimization experiments of adsorption conditions. 2.2. Optimization of Adsorption Conditions. 2.2.1. In-fluence of pH. The wastewater with an initial massconcentration of 10 g/L Cl was prepared, and the pH ofthe wastewater was adjusted to 5, 6, 7, 8,9, and 10. A total of 1g of adsorbent was:added to 100 mL of desulfurization Figure 3. Cremovalpercentages by the four modificationadsorbents. wastewater, which. was shaken for 2 hl at a constanttemperature of 40 °C in the water bath and then filtered.Figure 4 shows the removal percentage of Cl at different pHvalues. Figure 4. Effect of pH on adsorption rate. The points of zero charge (pHpzc) of fly ash, hydrotalcite,and the alkali-combined roasting-modified fly ash hydrotalciteare 4.86, 11.21, and 8.52, respectively.31,32 As can be seen fromFigure 4, initially, the removal percentage of Cl increasedsignificantly with pH. The removal percentage reached themaximum at pH 8 and then decreased with the increase in pH.Because fly ash hydrotalcite is alkaline. In an acidicenvironment, fly ash can react chemically and destroy theporous structure. The pH value of modified fly ash hydrotalciteadsorbent cannot be less than 4 because its interlayer structureis destroyed and its adsorption performance is reduced. In astrongly alkaline environment, the OH in the solutioncombines with part of the adsorption point when the pH isconsiderably high, and competitive adsorption occurs with Cl,reducing the dechlorination rate. 2.2.2.Influence of Temperature. Figure 5 shows that 1 g ofadsorbent was placed in 100 mL of desulfurization wastewaterwith an initial mass concentration of 10 g/L of Cl, and the pHof the waste solution was adjusted to 8. The solution was Figure 5. Effect of temperature on the adsorption rate. shaken in a water bath at 20, 30, 40,50, 60, 70, and 80°C for 2h and then filtered. The results show that the removalpercentage of Cl increased first and then decreased as theoscillation temperature increased. The removal percentagereached the maximum value at 60 °C. This result shows thatthe removal percentage of ions increases with the temperature.This finding may be attributed to the physical adsorption atlow temperatures and the temperature increase for easydesorption, thereby decreasing the removal percentage. Thechemical adsorption, adsorption site activity, and removalpercentage will all increase at 30-60 C. The molecularthermal movement will be violent when the oscillationtemperature continues to rise. This phenomenon results inthe easy desorption of adsorbed particles, which are notconducive to adsorption and decreases the removal percentage. 2.2.3. Influence of Input and Dosage. Modified fly ashhydrotalcite at 0, 0.2, 0.4, 0.6,0.8, 1, 1.2, and 1.4 g was addedto 1100 mL of wastewater containing the initial massconcentration of 10 g/L Cl at pH 8. The water bath wasmaintained at a constant temperature of60 °C for 2 h and thenfiltered. Figure 6 shows the effect of different amounts of flyash on Cl removal percentages in desulfurization wastewater. Figure 6. Effect of adsorbent dosage on the adsorption rate. Figure 6 shows that with the increase in modified fly ashadditive quantity, the Clremoval percentage continues to risewith the additional mass concentration of 10 g/L modified flyash. The removal percentage no longer increased when theadsorbent additive mass concentration continued to increase to14 g/L. This phenomenon may be observed when the Cl concentration in thee water for the reduction of theconcentration gradient is no longer evident and the diffusionflux is close to 0. Thus, modified fly ash adsorbent adsorptionreaches an equilibrium of Cl. 2.2.4. Influence of Shaking Time. A total of1 g ofadsorbent was added to 100 mL of desulfurization wastewaterwith 10 g/L initial mass concentration of Cl at pH 8, and theshaking time was set as 60, 120, 180, 240, and 300 min at anoscillation temperature of 60 °C. Figure 7 shows the effect ofdifferent shaking times on the Cl removal percentages indesulfurization wastewater. Figure 7. Effect of shaking time on adsorption rate. Figure 7 shows that the general trend of the Cl removalpercentage increased after the first reduction as the adsorptiontime increased and reached the maximum at 180 min. Theremoval percentage slightly dropped after 180 min. The resultsshow that increasing the stirring time can raise the contact timeand improve the adsorption effect. However, the adsorptionamount reached saturation due to a long time. At thistemperature, increasing the stirring time will not increase theadsorption rate. Thus, the removal percentage is reduced.Prolonged adsorption time is not conducive to the removal ofCl. Thus, the optimum shaking time is 180 min. Therefore, the optimum preparation conditions are asfollows: modification method using alkali-combined roasting(the ratio of fly ash to hydrotalcite is 2:1), mass concentrationof fly ash of 10 g/L, constant-temperature shaking time ofwater bath of 180 min, initial mass concentration of Clof 10g/L, oscillation temperature of 60 °C, waste liquid pH of 8,and adsorption rate of up to 68.1%. 2.3. Adsorption Isotherm. Adsorption isotherm refers toa curve that reflects the relationship of the amount of solute Qadsorbed on thee unit mass by adsorbents at constanttemperatures in a certain volume of solution and thecorresponding equilibrium concentration of the solution C.Adsorption isotherms can be used to describe the adsorptioncharacteristics of the adsorbents. The Freundlich andLangmuir adsorption isotherms are two widely used adsorptionisotherms in environmental chemistry. (1) Freundlich empirical formula where is the adsorption capacity of the adsorbent, mg/g;Cis the equilibrium concentration, mg/L; 1/n is the nonlinearcoefficient (1/n is; a constant describing the adsorption strength);0.1 <1/n <0.5 is easily adsorbed, 1/n >2 is difficultto adsorb; and k is the Freundlich constant. The above equation is usually expressed as a logarithmicequation of a line. (2) Langmuir empirical formula where Qis the adsorption capacity of the adsorbent, mg/g; C.is the equilibrium concentration, mg/L; Xm is the saturatedadsorption of adsorbent, mg/g; and b is the constant related toadsorption energy.Figure 8 shows the adsorption isotherms of Figure 8. Adsorption isotherm of Cl by the modified adsorbent. Figure 9. Freundlich isotherm model. modified fly ash and hydrotalcite at 25C. Figures 9 and 10show the Freundlich and Langmuir adsorption isotherms,respectively. The Langmuir model represents monolayeradsorption at specific homogeneous sites, while the Freundlichequation applies to multilayer adsorption. Both models reflecta certain temperature in a certain solution volume and theamount of solute Qby adsorbent adsorption on the unit masssolution corresponding to the equilibrium concentration of C.The adsorption characteristics of modified fly ash hydrotalciteat 25 °C were analyzed. According to the slope and intercept,the Freundlich constant 1/n at 25°C is 0.67325.1/n and Figure 10. Langmuir isotherm model. exceeds the range of 0.1-0.5. This result indicates that themodified fly ash hydrotalcite at 25C does not easily adsorbhigh concentrations of Cl,which is consistent with the single-factor variables discussed above. Figures 9 and 10 show thatthe correlation coefficient R² was 0.9826 and 0.9931,respectively. The adsorption isotherm was consistent withthe Langmuir isotherm model. The Langmuir modelrepresents the monolayer adsorption at specific homogeneoussites, while the Freundlich equation applies to multilayeradsorption. Moreover, the adsorption characteristics ofmodified fly ash hydrotalcite are more consistent with theLangmuir isotherm model, indicating that the adsorption ofCl by modified fly ash belongs to monolayer adsorption. Theadsorption saturation is 694.4 mg/g. Table 2 shows theBrunauer-Emmett-Teller (BET) surface areas and theadsorption capacity of different adsorbents., Table 2. BET Surface Areas and Adsorption Capacity ofDifferent Adsorbents specific adsorption surface area capacity adsorbents (m/g) (mg/g) alkali + roasting-modified fly ash hydrotalcite 24.58 694.4 OBC1000-3-10 62.26 4.34 OBC1000-3-15 86.52 6.76 KOBC15 303.59 11.13 3. CONCLUSIONS The increase in chloride ion concentration in the desulfuriza-tion absorbent will decrease the desulfurization efficiency andaffect the gypsum quality. The effective removal of chlorine ionfrom the desulfurization slurry is of great significance for thestable operation of the desulfurization system. Modified fly ashhydrotalcites were prepared by alkali/acid-combined roastingand microwaving and used as adsorbents of chlorine ion in thedesulfurized wastewater. The optimum preparation conditionsfor modifying fly ash hydrotalcite adsorbent are as follows:joint alkali-roasting modification method (fly ash and hydro-talcite ratio is 2:1), adsorbent concentration of 10 g/L, waterbath temperature shaking time of 180 min, Cl initial massconcentration of 10 g/L, oscillation temperature of 60C,liquid optimum pH of 8, and adsorption rate reaching 68.1%. Fly ash hydrotalcite adsorbent modified using joint alkaliroasting demonstrated the following: a large surface area andpore volume, a strong adsorption capability, consistent Cladsorption isotherm with the Freundlich1 and Langmuir adsorption isotherms, and correlation coefficient R of 0.9826and 0.9931. 4. MATERIALS AND METHODS 4.1. Preparation of Modified Fly Ash Hydrotalcite.The fly ash used in the experiment was obtained from theBaoding power plant. The chemical composition of thesamples was determined using the test method for the analysisof the coal ash according to the Chinese standard GB/T 1574-2007, as shown in Table 3. Table 3. Chemical Composition of Fly Ash chemical composition content (%) Sio, 54.1 Fez0s 17.5 Al,O3 11.6 CaO 11.3 TiO, 2.69 K,O 1.18 MgO 0.192 BaO 0.162 MnO 0.126 others 1.15 Table 3 shows that the contents of SiO, FezO3, and Al Oin fly ash account for 80%. Fly ash contains a large number ofirregular and closed pores rich in active vitreous particles, suchas SiO and Al,05. These vitreous particles contain manysmall active channels, thus providing a large specific surfacearea and good activity and increasing their adsorption capacity.The typical molecular composition of talc in the experiment isMgAl(OH)16COg·4H,O, also known as layered bimetallichydroxide, which is a kind of anion clay with a layeredstructure similar to the regular octahedral structure of bruciteMg(OH) 3535 4.2. Feature Analysis.. The wastewater used in theexperiment was simulated wastewater, which was dissolved inl L of deionized water with 10 g of NaCl, and the massconcentration of Cl" was 10 g/L. The main instruments usedin this experiment are listed in Table 5. The specific surfacearea and pore volume of the adsorbent were tested by theporous physical adsorption instrument of the American CantaEVO. The SEM images were obtained by field emission Table 5. Main Experimental Instruments the instrument model manufacturer microwave synthesis reac- XH-MC-1 Beijing Xianghu Technol- tion apparatus ogy Development Co., Ltd. muffle furnace SA2-9-17TP Nanyang Xinyu Furnace Industry Co., Ltd. vacuum drying oven ZDF6050 Shanghai Qixin Scientific Instrument Co., Ltd. speed-regulating multipur- HY-4 Changzhou Runhua Elec- pose oscillator tric Appliance Co., Ltd. ion meter PXS-270 Shanghai Electronic Sci- ence Instrument Co., Ltd. electronic balance FA2004B Shanghai Youke Instru- ment Co., Ltd. thermostatic magnetic HJ-6AB Changzhou Runhua Elec- stirrer tric Appliance Co., Ltd. BET (porous physical ad- QUANTACHROME Conta EVO sorption instrument) SEM (field emission scan- ning electron micro- JSM-7800F Prime Japanese Electronic JEOL scope) scanning electron microscopy and manufactured by JEOLElectronics in Japan. 4.3. Adsorption Experiment. A total of 100 mL ofsimulated waste liquid was added to 250 mL of the taperedflask. A total of 1 g of prepared adsorbent was also added, andthe tapered flask was placed onto a speed-regulating multi-purpose oscillator to vibrate for 60 min and then stopped. Thesimulated waste liquid was allowed to stand for a period andthen filtered. The clear liquid was taken to determine thecontent of Cl. The concentration of Cl was identifiedthrough the ion-selective electrode method. NaCl standard solution of 10-, 1, 5, and 10 g/L wasconfigured to draw the Cl standard curve. The abscissa of thestandard curve is the potential value, and the ordinate is thelogarithm of Cl-concentrations. The determination coefficientR is ≥0.999 based on the illustrated standard curve. Theparameters of the standard curve are shown in Figure 1. Theline equation in the standard curve illustration is obtained asfollows: y = kx+ b, where y is the potential value and x is thelogarithm of Clconcentrations, as shown in Figure 11. The linear regression equation is y=-53.835x+89.157.The correlation coefficient R²=0.9999. The concentration of simulated waste solution Co wasdetermined in the adsorption experiment, and the residualamount of Cl in the solution C was measured after theadsorption of modified fly ash. The adsorption amount ofmodified fly ash to Cl can be calculated by the followingformula adsorbent modification methods specific steps alkali-combined roast- ing modification Fly ash hydrotalcite was placed into 10 wt % sodium hydroxide solution to shake for 1 h and then filtered and roasted in a muffle furnaceat 450°C for 2 h to obtain adsorbent A. alkali combined with microwave modifi- cation Fly ash hydrotalcite was placed into 10 wt % sodium hydroxide solution to shake for 1 h and then filtered. Microwave irradiation wasconducted at 600 W, 60°C for 10 min and then maintained at room temperature for 2 h. Adsorbent B was obtained after cooling toroom temperature. C acid-combined roast- ing modification Fly ash hydrotalcite was placed into 10 wt % sulfuric acid solution to shake for 1 h and then filtered and roasted in a muffle furnace at450°C for 2 h to obtain adsorbent C. acid combined with microwave modifi- cation Fly ash hydrotalcite was placed into 10 wt % sulfuric acid solution to shake for 1 h and then filtered. Microwave irradiation was conductedat 600 W, 60°C for 10 min and then maintained for 2 h at room temperature. Adsorbent D was obtained after cooling to roomtemperature. Figure 11. Chloride ion standard curve. where Q is the equilibrium adsorption capacity, mg/g; Co isthe initial concentration, mg/L; C。 is the equilibriumconcentration, mg/L; m is theadsorbent mass, gi and V isthe solution volume, L AUTHOR INFORMATION Corresponding Authors Liqiang Qi - Department of Environmental Science andEngineering, North China Electric Power UniversityXBaodingCampus, Baoding 071003, P. R. China; @ orcid.org/0000-0001-6039-1224; Email: qiliqiang@ncepu.edu.cn Jingxin Li - Department of Environmental Science andEngineering North China Electric Power University区BaodingCampus, Baoding 071003, P. R. China; Phone: +8613933270460; Email: mutur@ncepu.edu.cn; Fax: +86-312-7525504 Authors Kunyang Liu - Department of Environmental Science andEngineering, North China Electric Power University区Baoding Campus, Baoding 071003, P. R. China Ruitao Wang - Department of Environmental Science andEngineering, North China Electric Power UniversityBaoding Campus, Baoding 071003, P. R. China Yajuan Zhang - Department of Environmental Science andEngineering, North China Electric Power University儿Baoding Campus, Baoding 071003, P. R. China Lan Chen - Department of Environmental Science andEngineering North China Electric Power University区Baoding Campus, Baoding 071003, P. R. China Complete contact information is available at:https://pubs.acs.org/10.1021/acsomega.0c04074 Notes The authors declare no competing financial interest. ACKNOWLEDGMENTS This work was supported by the Natural Science Foundationof Hebei Province: (Grant No. B2019502067) and theFundamental Research Funds for the Central Universities(Grant No.2020MS152). ( (1) Deng, X . ; Q i , L . Q . ; Z h ang, Y. J. E x perimental S t udy onAdsorption o f Hexavalent Chromium with Microwave-Assisted Alkali Modified Fly Ash. Water, Air, Soil Pollut . 2018, 229,17-22. ) ( (2) G itari, W. M.;P e trik, L. F.; Etchebers, O .; K ey, D. L.; Okujeni,C. U tilization of fl y ash for treatment of coal mines wastewater:Solubility controls on m ajor inorganic c o ntaminants. Fu e l 20 0 8, 87, 2450-2462. ) ( (3) Jiang, D . N.;Sha, H. W.; Go n g, G. H.; Song , J.; Xu, H. T.; Zhou, C. C.; Shen, K. Fundamental R esearch and Demonstration Project ofEvaporation Treatment of Wastewater from FGD in Flue Gas Duct. Adv. Mater. Res . 2014, 864-867, 4 34-437. ) ( ( 4) M a, S.; Chai, J.; Chen, G. D. ; Yu , W. J.; Zhu , S. J. Research ondesulfurization wast-ewater evaporation: P resent a nd future p erspec- tives. Renewable Sustainable Energy Rev . 2016 , S8 , 1143-1151. ) ( (S) Qi, L. Q; Li, J. T . ; Yao, Y.; Zhang, Y. J. Heavy Metal Poisonedand Re generation o f Selective Catalytic R e duction Catalysts. J. Hazard. Mater. 2019, 366, 4 92-500. ) ( (6) Nduta, K . G.; M wangi, I. W .; W anjau, R. W .; Ngila, J. C. Removal o f C hlorine and Chlorinated Organic Compounds f rom Aqueous M e dia Us i ng Su b strate-Anchored Z e r o-Valent B i metals. Water, A ir, Soil Pollut. 2016, 227, 10. ) ( ( 7) P eretyazhko, T. S . ; Ralston, S . J .; Sutter, B.; Ming, D. W .Evidence for A dsorption o f C h lorine Species on Iro n (II I )(Hydr)oxides i n the S heepbed M u dstone, Ga l e Cra t er, Mars. J. Geophys. Res. 2020, 12S, No. e2019JE006220. ) ( ( 8) W ang, X . X . ; S h en, X . D . Ex p erimental study on st r ength of lightweight aggregate c oncrete with different amounts of fly ash.Silicate Bull. 2011, 30, 69. ) ( (9) Z h ang, P. ; Li, M . Y. ; Lv , C. J.; Zhang, Y. J.; Wa n g, L. M.; F u, D . Effect of partial pressur e on CO a b sorption p e rformance inpiperazine promoted 2 - diethylaminoethanol and 1- d imethylamino-2- propanol aqueous s o lutions. J. C hem. Th ermodyn. 2 0 20, 150,No. 106198. ) ( (10) Qi, L . Q .; Y ao, Y.; H an, T . Y.; Li, J. T . Research o n the electrostatic c h aracteristic of coal-fired fly ash. Env i ron. Sci. Poll u t. Res. 2019,26,7123-7131. ) ( ( 11) Shi, D. Z.; Ma,J. Y.; Wang, H. L . ; Wang, P.; H u , C. Y.; Z h ang,J. L.; Gu, L . Detoxification of PCBs in fly ash from MSW incinerationby h ydrothermal t reatment with composite silicon-aluminumadditives and s eed i n duction. F u el P ro cess. Technol. 2019, 195, No. 106157. ) ( ( 12) Ma, L.J.; Han, L. N.; Chen, S . ; Hu, J. L.; Chang, L. P.; Bao, W.R.; Wang, J. C. Rapid synthesis o f magnetic zeolite materials from flyash and iron-containing wastes using supercritical water for elementalmercury removal from flue gas. F u el P r ocess. T echnol. 2 019, 189, 39- 48. ) ( (13) Wang, T. F. ; F a n, H. M. ; Ya n g, W. P.; Men g , Z. Stab i lizationmechanism of fly a s h t h ree-phase f o am an d its se a ling capacity onfractured reservoirs. Fuel 2020, 264, No. 1 16832. ) (14) Wang, S. B.; Ma, Q; Zhu, Z. H. Characteristics of coal fly ashand adsorption application. Fuel 2008, 87, 3469-3473. (15) Mohan, S.; Gandhimathi, R. Removal of heavy metal ions frommunicipal solid waste leachate using coal fly ash as an adsorbent.J.Hazard. Mater. 2009, 169, 351-359. (16) Chatterjee, A.; Hu, X. J.; Lam, F. L. Y. Modified coal fly ashwaste as an efficient heterogeneous catalyst for dehydration of xyloseto furfural in biphasic medium. Fuel 2019, 239, 726-736. (17) Wang, N.; Sun, X. Y.; Zhao, Q; Wang, P. Treatment ofpolymer-flooding wastewater by a modified coal fly ash-catalysedFenton-like process with microwave pre-enhancement: Systemparameters, kinetics, and proposed mechanism. Chem. Eng. J. 2021,406,No. 126734. (18) Wang, S. B.; Boyjoo, Y.; Choueib, A.; Zhu, Z. H. Removal ofdyes from aqueous solution using fly ash and red mud. Water Res.2005, 39, 129-138. (19)Nguyen, T. C.; Tran, T. D. M.; Dao, V. B.; Vu, Q. T.; Nguyen,T. D.; Thai, H. Using Modified Fly Ash for Removal of Heavy MetalIons from Aqueous Solution. J. Chem. 2020, 2020, No. 8428473. (20) Sahoo, P. K.; Tripathy, S.; Panigrahi, M. K.; Equeenuddin, Sk.Md. Evaluation of the use of an alkali modified fly ash as a potentialadsorbent for the removal of metals from acid mine drainage. Appl.Water Sci. 2013, 3, 567-576. ( (21 ) Li, P . L .; Hu, Z .; Wang, C . X.; W a ng, C. L.; Hu , F. M . Stu d y on the p reparation o f acid-modified f l y ash a n d its degradation of b eneficiation wastewater COD. Multipurp. Util. Mi n er. Re s our. 20 1 9, 02, 103-108. ) (22) Malarvizhi, T.S.; Santhi, T. Lignite fired fly ash modified bychemical treatment for adsorption of zinc from aqueous solution. Res.Chem. Intermed. 2013, 39,2473-2494. (23) Zhang, F.; Ou, Y. P.; Zhang, X. M.; Chen, L. Research progresson modification and adsorption application of fly ash. Appl. Chem. Ind.2016,45, 747-750. ( (24) Q i, L . Q .; T eng, F . ; Deng, X . ; Z h ang, Y. J.; Z h ong, X. Y. Experimental study on adsorption of Hg(II) with m i crowave-assistedalkali-modified fly as. Powde r Technol. 2019, 351 , 153-158. ) ( (25) An, C . J.; Y ang, S . Q.; Huang, G. H.; Zha o , S.; Z hang, P.; Yao, Y . Removal of sulfonated humic acid from aqueous phase by modified coal fly ash waste: Equilibrium and k inetic adsorption s tudies. Fuel2016, 165,264-271. ) ( (26) S hi, Y. F.; Wang, X . H .; Z hang, L. J.; Li, Z. H. Study on theremoval performance of hydrotalcite and fly ash on Cland SO- indesulfurization wastewater. Bull . Chin . Ceram. Soc. 2015, 34, 1402- 1406. ) ( (27) A lonso-de-Linaje, V.; Mangayayam, M. C. ; To b ler, D. J; Dietmann, K . M . ; R s pinosa, R . ; Rivers, V .; D alby, K. N. Sorption o f chlorinated hydrocarbons from s y nthetic and natural groundwater by organo-hydrotalcites: Towards t h eir a p plications a s remediation nanoparticles. Chemosphere 2019, 236, No. 124369. ) ( (28) L i , D. X . ; C hen, Y. M.; Shen, J.L.; Wa n g, Y. J.; Su , J. H. Ef f ectsof alkali on activation a nd microstructure of fly ash. J. Mater. Sci. Eng. 2000, 01, 5 2. ) ( (29) F ang, J . L .; L u, W . X . ; X u , C . X. Pr o gress in ac t iviting t echniques and m e chanism stud i es of f l y ash . J. Shanghai Uni v . (Na t .Sci. Ed.) 2002,8 , 255-260. ) ( (30) O u, Y. P.; Fan, H. Y.; Zhang, X. M.; Chen, L. R esearch progress on t he mechanism of fl y a s h m o dification b a sed o n adsorption. J. Mater. Sci. Eng. 2014, 32,619-624. ) ( (31) K a ranac, M.; Do l ic, M. ; Veljovic, D.; Rajakovic-Ognjanovic, V.; Velickovic, Z.; Pavicevic, V. ; Marinkovic, A. The r emoval o f Z n , P bt, an d As(V) ion s by lime acti v ated fly ash and v alorization of theexhausted adsorbent. Waste Manage. 2018, 78, 366-378. ) ( (32) S hah, B . A .; Oluyinka, O. A .; Shah, A. V. F ly Ash R euse a s Mesoporous C a - a n d Mg-Zeolitic C o mposites fo r the Seclusion o f Aniline from Aqueous So l ution. Arab. J. S c i. Eng. 2019, 4 4, 289-304. ) ( (33) Guan, C. H.; Lv, X. J.; H an, Z. X.; Chen, C . ; X u , Z . M . ; L i u, Q . S. The adsorption enhancement of graphene for fluorine and chlorine from water. Appl. S urf. Sci. 2020, S16, No. 1 46157. ) ( (34) D u, Z. Y.; T ian, W . J.; Qiao,K . L . ; Zhao,J. ; Wang, L. ; Xie, W . L.; Chu, M. L.; Song, T.T. Improved chlorine and chromium i onremoval f rom leather p rocessing wastewater b y biocharcoal-basedcapacitive d eionization. S ep. P urif. Technol. 2020, 233, No. 11 6 024. ) ( (35) C avani, F . ; T rifiro, F.; Vaccari, A. Hydrotalcite-type anionicclays: Preparation, properties and applications. Catal. Today 1991, 1 1, 173-301. ) https://dx.doi.org/acsomega.cCS Omega XXXX, XXX,XXX-XXXACS Publications O XXXX American Chemical SocietyA Bhttps://dx.doi.org/acsomega.cCS Omega XXXX, XXX, XXX-XXX 利用改性粉煤灰氢氧化铝去除脱硫废水中的氯离子改性粉煤灰氢氧化铝 通过碱/酸混合焙烧和微波制备,并将其作为氯离子的吸附剂。用作脱硫废水中氯离子的吸附剂。通过X射线衍射(XRD)和扫描电子显微镜(SEM)分析了不同吸附剂的比表面积和孔隙率。显微镜(SEM)。研究了pH值、温度、吸附剂用量和吸附摇动时间对吸附性能的影响进行了研究。结果显示,碱合焙烧改性的粉煤灰氢氧化铝对Cl-具有最佳的去除效果。1. 简介 脱硫废水中含有大量的悬浮固体(如石膏颗粒、二氧化硅、铝和氢氧化铁)、无机盐和微量重金属,如:砷、镉、铬和汞,因此,如果直接排放,会严重危害环境。如果直接排放,会对环境造成危害。氯化物在烟气中的溶解会增加脱硫剂中的氯离子浓度。这种增加会降低脱硫率并影响石膏的质量。从而导致脱硫系统的腐蚀。在经过 "中和、凝结、沉淀 "的三箱过程后。脱硫系统中的重金属和悬浮物的含量就会降低。但氯的含量仍然很高。2017年火电厂污染防治可行技术指南(HJ2301-2017)指出 实现废水近零排放的关键是实现脱硫废水的零排放。目前用于去除废水中Cl-的主要方法有电凝法、离子交换法和吸附法。 吸附法被认为是从废水中吸收Cl-的有效方法之一。因为它具有操作简单、可回收等优点。粉煤灰是火力发电站的副产品。中国是一个煤炭的生产和使用大国。中国的燃煤发电每年可产生数以亿计的粉煤灰。因此,粉煤灰在处理废水中的有害物质方面,具有成本低、易获取、多孔物理组织、比表面积大、吸附活性高等特点。因此,利用粉煤灰处理废水中的有害物质已被广泛研究。粉煤灰从废水中去除磷的研究始于1980年,当时Kuziemska使用粉煤灰作为廉价的吸附剂来处理城市固体废物浸出物中的重金属离子。研究表明,改性粉煤灰具有更大的比表面积和更强的吸附能力。因此,使用改性粉煤灰来处理一些有害物质是可能的 由于其强大的吸附性能。Wang17使用H2SO4浸渍法来改性原煤粉煤灰(RCFA),并将改性后的RCFA(ACFA)作为芬顿类催化剂,微波预强化条件下处理聚合物浸泡废水。粉煤灰在室温下被硝酸改性。改良后的粉煤灰对亚甲基蓝的吸附性能显著提高。使用2-巯基苯并噻唑(MBT)和多硫酸钠(SDS)作为表面活性剂,并将粉煤灰用微波预强化处理。表1. 比表面积和孔隙体积分析图2. 碱改性飞鸟的飞机布局a图3. 四种改性的Cl-去除率吸附剂。图4. pH值对吸附率的影响图5. 温度对吸附率的影响。图6. 吸附剂用量对吸附率的影响图7. 摇动时间对吸附率的影响。图8. 改性吸附剂对Cl-的吸附等温线。图9. Freundlich等温线模型。图10. Langmuir等温线模型。2. 结论 脱硫吸收剂中氯离子浓度的增加会降低脱硫效率并影响石膏的质量。有效去除脱硫浆液中的氯离子,硫系统的稳定运行具有重要意义。改性粉煤灰通过碱/酸混合焙烧和微波处理制备了改性粉煤灰氢氧化铝。并将其作为脱硫废水中氯离子的吸附剂。改造粉煤灰氢氧化铝吸附剂的最佳制备条件如下。 联碱焙烧改性法(粉煤灰和氢氧化铝的比例为2:1),吸附剂浓度为10g/L,水浴温度为180分钟。水浴温度晃动时间为180分钟,Cl-初始质量浓度为10g/L,振荡温度为60℃。液体最佳pH值为8,吸附率达到68.1%。采用联合碱法改性的粉煤灰氢氧化铝吸附剂,焙烧法改性的粉煤灰氢氧化铝吸附剂表现出以下特点: 较大的表面积和孔隙体积,较强的吸附能力,与Cl-吸附等温线与Freundlich和Langmuir吸附等温线一致,相关系数R2为0.9826和0.9931。
关闭-
1/8

-
2/8
还剩6页未读,是否继续阅读?
继续免费阅读全文北京祥鹄科技发展有限公司为您提供《脱硫废水中利用改性粉煤灰氢氧化铝去除氯离子检测方案(微波合成仪)》,该方案主要用于废水中无机阴离子检测,参考标准《暂无》,《脱硫废水中利用改性粉煤灰氢氧化铝去除氯离子检测方案(微波合成仪)》用到的仪器有祥鹄实验室微波合成反应仪、微波催化合成萃取仪 。
我要纠错
相关方案







 咨询
咨询







