方案详情文
智能文字提取功能测试中
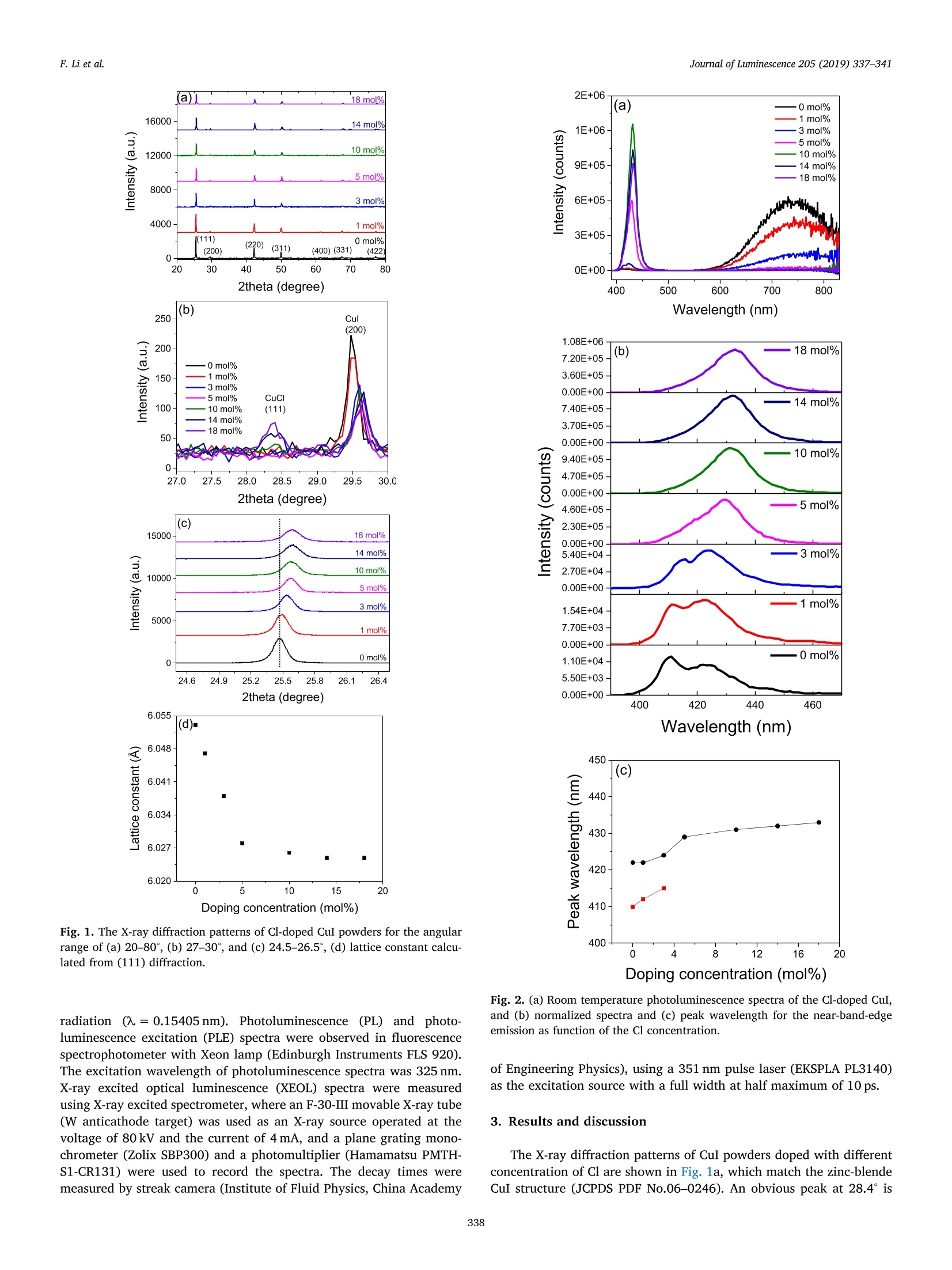
Journal of Luminescence 205 (2019) 337-341Contents lists available at ScienceDirect F. Li et al.Journal of Luminescence 205 (2019) 337-341 Journal of Luminescence journal homepage: www.elsevier.com/locate/jlumin Enhancement of the near-band-edge emission of CuI by Cl doping Fengrui Li, Mu Gu*, Xiaolin Liu , Shuangqiang Yue, Jiajie Zhu, Qianli Li, Yahua Hu,Shiming Huang, Bo Liu, Chen Ni Shanghai Key Laboratory of Special Artificial Microstructure Material & Technology, School of Physics Science and Engineering, Tongji University, Shanghai 200092,China College of Materials Science and Engineering, Shenzhen University, Shenzhen, Guangdong 518060, China ARTICLEIN F O ABSTRACT Keywords:CuICl dopingNear-band-edge emissionIsoelectronic bound exciton CuI is a promising candidate for scintillation devices due to the ultrafast decay time of 130 ps at room tem-perature. The photoluminescence of CuI is composed of the near-band-edge emission (fast decay) and the deep-level emission (slow decay). Unfortunately, the fast component is much weaker than the slow component. Theeffect of Cl doping on the fast and slow components is investigated in this paper to improve the luminescent andscintillating properties. X-ray diffraction patterns show that Cl ions are successfully doped into CuI lattice for lowconcentration (< 10 mol%), whereas the CuCl phase is formed for high concentration. The Cl doping formsisoelectronic bound excitons and enhance near-band-edge emission and suppress the deep-level emission, whichis demonstrated to be stable for long time. Furthermore, the near-band-edge emission decay times of CuI hardlychange with Cl doping. 1. Introduction Cuprous iodide (CuI) is a wide band gap intrinsic p-type semi-conductor (3.115 eV,T= 4.2K) [1] with a large exciton binding energy(62 meV) [2], and a high hole mobility (43.9 cm²v-1s-) [3]. It canbe used in electrochemistry and energy storage devices, such as trans-parent electrodes and solar cells [4-6]. Recently, CuI has drawn muchattention due to the fast decay time of 130 ps at room temperature [7],showing promising potential in ultrafast radiation detection [8]. The photoluminescence of CuI consists of the near-band-edgeemission (NBE) and deep-level emission (DLE). The near-band-edgeemission is composed of several bands. A sharp band with a peak at410 nm (room temperature) originates from the recombination of freeexcition [9]. The mechanism of the band at about 420-430 nm is stillnot clear. Nikitenko et al. has studied excitation dependence of thephotoluminescence of CuI single crystal [10]. The blue shift of the bandat 431 nm with increasing excitation level at 80 K indicates that theemission originates from the recombination of donor-acceptor pairs.Perera et al. believe the band at 417 nm is ascribed to the recombina-tion of the electron in the conduction band and the trapped hole causedby absorbed iodine [11]. Xia et al. deduce that the band at 422 nmoriginates from the Cu vacancies based on the luminescent properties ofthe CuI film deposited on Cu substrates [12]. On the other hand, thedeep-level emission shows a broad band at 680-720nm, which is attributed to the I vacancies [13,14]. However, the decay time of thedeep-level emission is found to be slow (2.82 us) [15]. Although theslow component is much more intensive than the fast component, theslow component is unfavorable for the application in ultrafast scintil-lation devices. Therefore, it is necessary to enhance the fast componentand suppress the slow component. Although iodine annealing can enhance the near-band-edge emis-sion and suppress the deep-level emission of CuI films, the effect dis-appears quickly due to instability of the iodine on the surface [12,16].Zn doping has been demonstrated to enhance the emission at 422 nmbecause of the creation of donors, but the effect of Zn doping on thedeep-level emission is not known [17]. Nowadays, the luminescentmechanism of CuI is still not clear in comparison to other famoussemiconductors such as ZnO [18-20] and GaN [21,22]. Motivated bythe results of iodine annealing, we perform Cl doping in CuI powder inthis paper to improve the luminescent and scintillating properties. 2. Experimental Analytical reagent grade CuI and CuCl were purchased fromSinopharm Chemical Reagent Co., Ltd. The mixture of CuI and CuClpowders with different ratio was heated to 460℃ and then kept for30 min in a tube furnace in Ar atomosphere. X-ray diffraction wasperformed using X-ray diffractometer (Haoyuan DX-2700) with Cu K ( E-mail address: mgu@tong j i . edu.cn ( M. Gu). ) ( h t t ps://doi.org/ 1 0.1016/j . jlumin . 2018.09.040 ) ( Received 13 M arch 2018; Received in r e vised form 20 J u ly 2018; Accepted 19 September 2018Available online 19 September 2018 ) ( 0022-2313/C 2018 Elsevier B.V. All ri g hts reserved. ) Doping concentration (mol%) Fig. 1. The X-ray diffraction patterns of Cl-doped CuI powders for the angularrange of (a) 20-80°, (b) 27-30°, and (c) 24.5-26.5°, (d) lattice constant calcu-lated from (111) diffraction. radiation (A=0.15405nm). Photoluminescence (PL) and photo-luminescence excitation (PLE) spectra were observed in fluorescencespectrophotometer with Xeon lamp (Edinburgh Instruments FLS 920).The excitation wavelength of photoluminescence spectra was 325 nm.X-ray excited optical luminescence (XEOL) spectra were measuredusing X-ray excited spectrometer, where an F-30-III movable X-ray tube(W anticathode target) was used as an X-ray source operated at thevoltage of 80 kV and the current of 4 mA, and a plane grating mono-chrometer (Zolix SBP300) and a photomultiplier (Hamamatsu PMTH-S1-CR131) were used to record the spectra. The decay times weremeasured by streak camera (Institute of Fluid Physics, China Academy 2E+06 (a) -0mol% ·1 mol% 1E+06 ·3mol% 0 4 8 12 20 Doping concentration (mol%) Fig. 2. (a) Room temperature photoluminescence spectra of the Cl-doped CuI,and (b) normalized spectra and (c) peak wavelength for the near-band-edgeemission as function of the Cl concentration. of Engineering Physics), using a 351 nm pulse laser (EKSPLA PL3140)as the excitation source with a full width at half maximum of 10 ps. 3. Results and discussion The X-ray diffraction patterns of CuI powders doped with differentconcentration of Cl are shown in Fig. la, which match the zinc-blendeCuI structure (JCPDS PDF No.06-0246). An obvious peak at 28.4°is -77K (a) 0 mol% 100 K 1E+07· ·77K (c) 10 mol% 100K 200 250 300 350 400 Wavelength (nm) found for the Cl concentration of 14 mol% and 18 mol%, as shown inFig. 1b, due to the formation of an isostructural CuCl phase (JCPDS PDFNo.06-0344). The peak of the (111) plane shifts to larger angles withincreasing Cl concentration because of different ionic radius between Cland I, see Fig. 1c. Furthermore, the shift of the peak becomes saturatedfor the Cl concentration above 10%, reflecting limited solubility of Cl in CuI lattice, due to different electronegativity between Cl and I [23].Incorporation of Cl in CuI lattice is confirmed by the lattice constantscalculated from the diffraction peaks of the (111) plane, as shown inFig. 1d. The room temperature photoluminescence of the Cl-doped CuIpowder samples are mainly composed of the near-band-edge emission and the deep-level emission as shown in Fig. 2a. The deep-level emis-sion related to the I vacancies is suppressed by Cl doping, reflecting thepossibility of filling the vacancies by Cl. On the contrary, the near-band-edge emission is enhanced and reaches the maximal intensity for the Clconcentration of 10 mol%. In order to understand the mechanism of the near-band-edgeemission, we measure the temperature dependence of the emissionspectra for the undoped (0 mol%) and 10 mol% Cl-doped CuI powdersamples as shown in Fig. 3. In the case of undoped sample two bandswith peaks at 406 nm and 428 nm are found at 77 K. The recombinationof the free exciton is considered to be responsible for the band at406 nm [10]. The intensity of the band at 428 nm decreases graduallywith increasing temperature together with a blue shift, see Fig. 3a andb. Since the donor-acceptor pair with large distance (weak Coulombinteraction) has high thermal dissociation probability, only the pairswith small distance (strong Coulomb interaction) contributes to theemission at high temperature, leading to the shift of the emission to ahigher energy [24]. Although several possible mechanisms, such as thedonor-acceptor pairs [10] and the absorbed iodine [11], have beenproposed to explain the origin of the band at 428 nm, our results sup-port the recombination of the donor-acceptor pairs. A more intensive band at 429 nm is observed for the 10 mol% Cl-doped sample with a different temperature dependence feature ascompared to the undoped sample as shown in Fig. 3c and d. In addition,the photoluminescence excitation spectra (Fig.3e) show peaks at 217and 250 nm for undoped sample, whereas the peak at 250 nm is sup-pressed for the Cl-doped sample together with two growing peaks at280 nm and 370 nm. The results indicate that the mechanism of thenear-band-edge emission for the Cl-doped CuI is different from the re-combination of the donor-acceptor pairs. Isoelectronic defects can beformed due to the difference of electronegativity and radius between Cland I. Excitons can be localized due to the interaction between theelectrons and the traps [25] and thus the recombination of the iso-electronic bound excitons enhances the emission. The near band edgeemission intensities of Cl-doped CuI increases with increasing isoelec-tronic bound excitons, until the Cl becomes saturated in CuI. Besides,the suppression of iodine vacancies by Cl doping may also lead to theimprovement of near band edge emission intensity, since the near bandedge emission and deep level emission is competitive [16]. The re-duction of emission intensity implies that the CuCl impurity may en-hance the non-radiative recombination process, when the Cl con-centration is above 10 mol% in CuI [26]. The emission spectra at roomtemperature shows two peaks at 410 nm (free exciton) and 422 nm(donor-acceptor pairs) for the undoped sample (Fig. 2b). The peak offree exciton emission shows a red shift (Fig. 2c) with increasing Clconcentration (≤3 mol%), being ascribed to the band gap narrowingeffect induced by lattice distortion [27]. The free exciton emissionoverlaps the isoelectronic bound exciton emission introduced by Cldoping with the concentration above 3 mol%, which is demonstrated bythe non-Gaussian shape of the spectra. Since scintillators are used to detect high energy rays, the X-rayexcited optical luminescence spectra of Cl-doped CuI powder samplesare depicted in Fig. 4. The near-band-edge (deep-level) emission isenhanced (suppressed), which is consistent with that for the photo-luminescence. The integral intensity of 10 mol% Cl-doped sample is 5.9times as the commercial CuI powder. A red shift of the near-band-edgeemission is also found. Fig. 5a and b show the photoluminescence andX-ray excited optical luminescence spectra for 10 mol% Cl-dopedsample measured at different time to examine the stability of the Cldoping in CuI. The spectra is almost unchanged after 4 months, which ismuch better than for iodine annealing [12]. Besides, the near-band-edge emission decay times of undoped and 10 mol% Cl-doped CuI are22.3 ps and 22.7 ps, shown in Fig. 5c. This result indicates that the near-band-edge emission of Cl-doped CuI is still very fast. Fig. 4. (a) X-ray excited optical luminescence spectra at room temperature forCl-doped CuI, and (b) integral intensity and (c) peak wavelength of the near-band-edge emission as function of the Cl concentration. 4. Conclusion The luminescent and scintillating properties of Cl-doped CuI havebeen investigated. Cl doping leads to the shift of X-ray diffraction pat-terns. In the case of undoped sample the near-band-edge emission isascribed to the recombination of donor-acceptor pairs and shows a blueshift with increasing temperature. The excitation spectra and the shiftof the emission spectra for the Cl-doped sample are different from theundoped case, which may be related to the introduction of the iso-electronic bound excitons. The photoluminescence and X-ray excitedluminescence optical spectra show high stability after 4 months.Importantly, Cl doping turns out to suppress the deep-level emission(slow decay) and enhance the near-band-emission (fast decay) with anoptimum concentration of 10 mol%, leading to improvement of the Time (ps) Fig. 5. (a) photoluminescence and (b) X-ray excited optical luminescencespectra of 10 mol%Cl-doped CuI measured at different time,(c) near-band-edgeemission decay times of undoped (0mol%) and 10 mol% Cl-doped CuI, IRFrepresents instrument response function of the pulse laser and streak camera. luminescent and scintillating properties of CuI. And the near-band-edgeemission decay time of CuI is still very fast after Cl doping. Acknowledgements This work was supported by the National Natural ScienceFoundation of China (Grant nos. 11475128,11675121,11775160 and11375129) and Significant National Special Project of the Ministry ofScience and Technology of China for Development of ScientificInstruments and Equipment (Grant No. 2011YQ13001902). ( [1] T . Goto, T. Takahash i , M . Ue t a, E x c i t o n lu m inescence of C uCl , C u B r and CuI si n gle c rystals, J. P h ys. Soc. Jpn. 24 (1968) 3 14-327. ) ( [2] H . I c h i da, Y . Kanematsu, T . Shimomura, K. M izoguchi, D. Kim, M . N a kayama , P h otolumine s cence dyn am ics o f e x c iton-exciton scattering pr o cesses in CuI th i n films, P hy s. Rev . B 72 (4) (2005)045210. ) ( [3] 1 D . C h en, Y. W a ng, Z. L i n , J. H u a ng, X. C h e n , D. P a n, F . H u ang, G r owth s trategy an d p h y si c al p roperties of the hi g h mobi l it y P-T y pe C uI c rystal, Crys t . Growt h D es. 1 0(5) ( 2010) 2 057-2060. ) ( [4] M . G rundmann, F . S c hein, M . L or enz, T. Bontgen, J . L enzner, H . W enckstern, Cup r ous i o di d e - a p-ty p e t r ansparent s e m icon d ucto r : hi s tory and no v el applica - t ions, Phys . Status Solidi A 2 1 0 (2013 ) 1671- 1 703. ) ( [5] J . Chr i stian s , R . F u ng, P . K am a t, A n i n o rg a nic h ole conduct o r for organo-lead hali d e p erovskite solar c ells. I mproved ho l e conductivi t y w i th co pper io d i de , J . A m . C h e m . Soc.136(2) (2014)758-764. ) ( [6] 1 F . Schein , H . Wenckste r n , M . Grundmann,Tr a nsparent p-CuI/n - Z n O h eterojunction d iodes, A ppl. P hys. L e tt. 102 (9) (2013) 092109. ) ( [7] S . D erenzo, M . W e b e r, M. K l i n t enberg, Te m peratu r e dependence of the f ast, near- b and- e dge scintilla t ion from C u I, HgI2, Pb2 , ZnO:Ga an d C dS:In, Nucl. I nstr u m. M ethods Phy s . R es. Sect . A 486 (1)(2002) 2 1 4-219. ) ( [8] P . R odn yi , Pro gr ess i n f ast scintill a tors , Radia t . Meas. 3 3 (5) (2001 ) 605 - 614. ) ( [9] I . Vereshchagin, V . N ikitenko,S . S toyu k h in, B a nd-edge em i ssion in C uI single c ry sta l s, J . Lu min . 29 ( 1984) 215- 2 21 . ) [10]V. Nikitenko,S. Stoyukhin, V. Popolitov, Y. Mininzon, Luminescence of CuI singlecrystals, J. Appl. Spectrosc. 34 (1981) 410-413. [11]V\. Perera, K. Tennakone, Recombination processes in dye-sensitized solid-statesolar cells with CuI as the hole collector, Sol. Energy Mater. Sol. Cells 79 (2003)249-255 ( [12] 1 M . X i a, M . G u , X. L i u , B . L i u, S. H u ang, C. N i , L u minescence characteris ti cs of C uI f ilm b y i odine a nnealin g, J . M ater. Sci. Mater . Electron. 26 (2015) 1 - 5. ) [13]P. Gao, M. Gu, X. Liu, Y. Zheng, E. Shi, Crystal growth and luminescence propertiesof CuI single crystals, Optik 125 (2014) 1007-1010. [14]P1. Gao, M. Gu,X. Liu, Y. Zheng, E. Shi, Photoluminescence study of annealing ef-fects on CuI crystals grown by evaporation method, Cryst. Res. Technol. 47 (2012)707-712. [15] S. Saha, S. Das, D. Sen, U. Ghorai, N. Mazumder, B. Gupta, K. Chattopadhyay, Baneto boon: tailored defect induced bright red luminescence from cuprous iodide na-nophosphors for on-demand rare-earth-free energy-saving lighting applications, J.Mater. Chem. C 3 (2015) 6786-6795. [16]IP. Gao, M. Gu, X. Liu, B. Liu, S. Huang, X-ray excited luminescence of cuprousiodide single crystals: on the nature of red luminescence, Appl. Phys. Lett. 95 (2009)221904. [17] M. Xia,M. Gu, X. Liu, B. Liu, S. Huang, C. Ni, Electrical and luminescence properties of Zn2+doped CuI thin films, J. Mater. Sci. Mater. Electron. 26 (2015) 2629-2633. [18]J. Yoo, G. Yi, B. Chon, T. Joo, Z. Wang, Luminescence dynamics of bound exciton ofhydrogen doped ZnO nanowires, J. Lumin. 176 (2016) 278-282. [19]E. Kumar, F. Mohammadbeigi, L. Boatner, S. Watkins, High-resolution photo-luminescence spectroscopy of Sn-doped ZnO single crystals, J. Lumin. 176 (2016)47-51. [20]1Q. Li, X. Liu, M. Gu, S. Huang, J. Zhang, B. Liu, C. Ni, Y. Hu, S. Zhao, Q. Wu,Enhanced X-ray excited luminescence of Ga- and In-doped ZnO nanorods by hy-drogen annealing, Mater. Res. Bull. 86 (2017) 173-177. [21]] R. Garcia-Gutierrez, A. Ramos-Carrazco, D. Berman-Mendoza, G. Hirata,O. Contreras, M. Barboza-Flores, Photoluminescence enhancement from GaN byberyllium doping, Opt. Mater. 60 (2016) 398-403. [22]U1. Kaufmann, M. Kunzer, H. Obloh, M. Maier, C. Manz, A. Ramakrishnan, B. Santic,Origin of defect-related photoluminescence bands in doped and nominally undopedGaN, Phys. Rev. B 59 (1999) 5561-5567. [23]IM. Seong, H. Alawadhi, I. Miotkowski, A. Ramdas,S. Miotkowska, Role of elec-tronegativity in semiconductors: isoelectronic S, Se, and O in ZnTe, Phys. Rev. B 62(2000)1866-1872. [24] S. Lautenschlaeger, S. Eisermann, G. Haas, E. Zolnowski, M. Hofmann, A. Laufer,M. Pinnisch, B. Meyer, M. Wagner,J. Reparaz, G. Callsen, A. Hoffmann, A. Chernikov, S. Chatterjee, V. Bornwasser, M. Koch, Optical signatures of nitrogenacceptors in ZnO, Phys. Rev. B 85 (2012). [25]M. Seong, I. Miotkowski, A. Ramdas, Oxygen isoelectronic impurities in ZnTe:photoluminescence and absorption spectroscopy, Phys. Rev. B 58 (1998)7734-7739. [26]X. Wang, C. Song, K. Geng, F. Zeng, F. Pan, Photoluminescence and Raman scat-tering of Cu-doped ZnO films prepared by magnetron sputtering, Appl. Surf. Sci.253 (2007) 6905-6909. [27] B. Bouhafs, H. Heireche, W. Sekkal, H. Aourag, M. Certier, Electronic and opticalproperties of copper halides mixed crystal CuCl1-xIx,Phys. Lett. A 240 (1998)257-264. CuI is a promising candidate for scintillation devices due to the ultrafast decay time of 130 ps at room temperature. The photoluminescence of CuI is composed of the near-band-edge emission (fast decay) and the deeplevel emission (slow decay). Unfortunately, the fast component is much weaker than the slow component. The effect of Cl doping on the fast and slow components is investigated in this paper to improve the luminescent and scintillating properties. X-ray diffraction patterns show that Cl ions are successfully doped into CuI lattice for low concentration (< 10 mol%), whereas the CuCl phase is formed for high concentration. The Cl doping forms isoelectronic bound excitons and enhance near-band-edge emission and suppress the deep-level emission, which is demonstrated to be stable for long time. Furthermore, the near-band-edge emission decay times of CuI hardly change with Cl doping.
关闭-
1/5

-
2/5
还剩3页未读,是否继续阅读?
继续免费阅读全文产品配置单
北京欧兰科技发展有限公司为您提供《掺杂CI的CuI中CuI近边带发射检测方案(激光产品)》,该方案主要用于电子元器件产品中CuI近边带发射检测,参考标准《暂无》,《掺杂CI的CuI中CuI近边带发射检测方案(激光产品)》用到的仪器有PL3140 皮秒Nd:YLF激光器。
我要纠错
相关方案






 咨询
咨询




