方案详情文
智能文字提取功能测试中
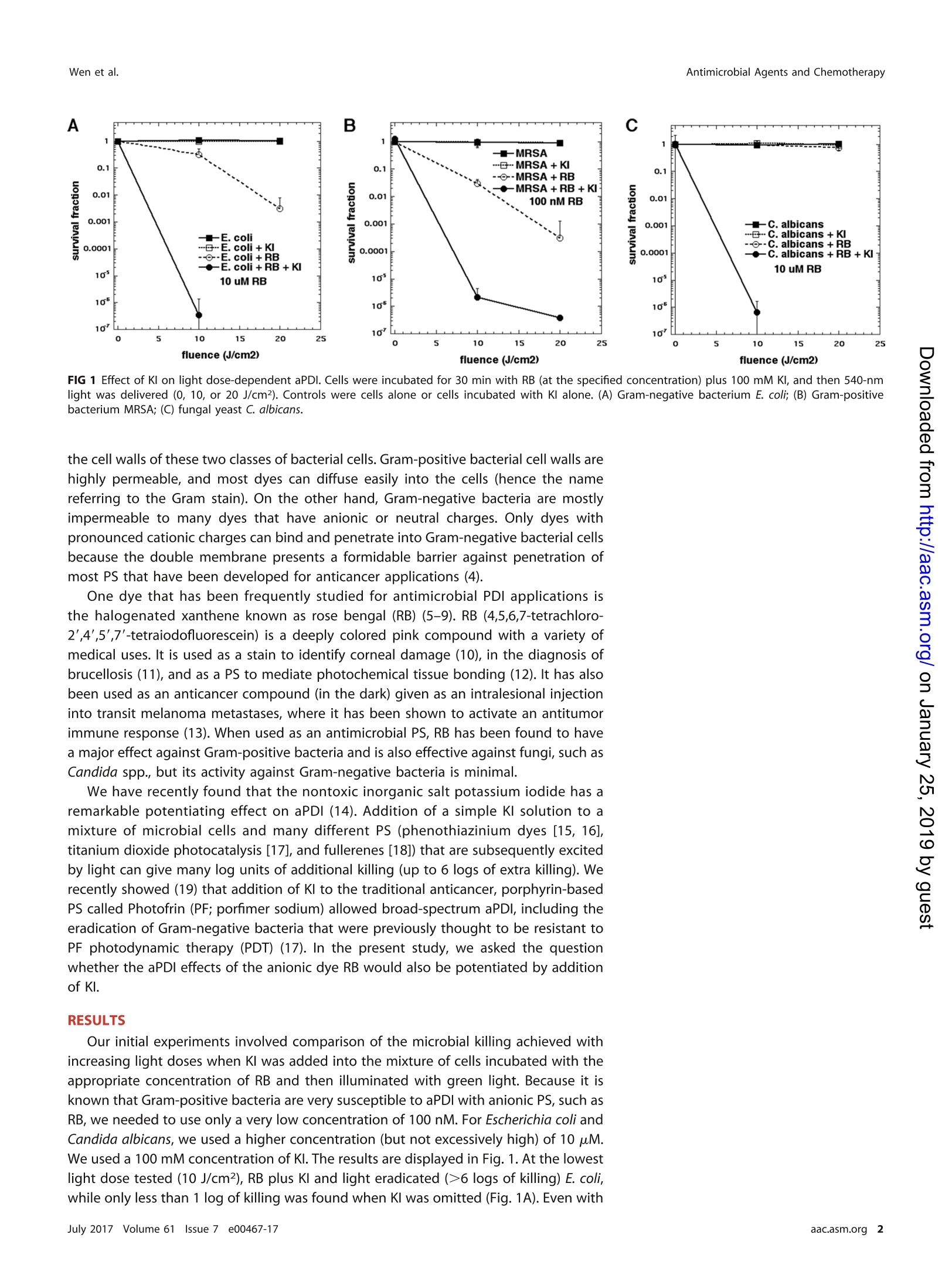
EXPERIMENTAL THERAPEUTICS Antimicrobial Agents and ChemotherapyWen et al. Potassium lodide Potentiates AntimicrobialPhotodynamic Inactivation Mediated byRose Bengal in In Vitro and In VivoStudies Xiang Wen,a,b,c Xiaoshen Zhang,b,d,e Grzegorz Szewczyk,f Ahmed El-Hussein,b,c,gYing-Ying Huang,b.c Tadeusz Sarna,ff DMichael R. Hamblinb.c,h Department of Dermatology, West China Hospital of Sichuan University, Chengdu, Sichuan, China; WellmanCenter for Photomedicine, Massachusetts General Hospital, Boston,Massachusetts,USAb;Department ofDermatology, Harvard Medical School,Boston, Massachusetts,USA; Department of Cardiology, ShanghaiTenth Hospital of Tongji University,Shanghai, Chinad;Tongji University Cancer Institute, Tongji UniversitySchool of Medicine, Shanghai, China; Department of Biophysics, Faculty of Biochemistry, Biophysics andBiotechnology, Jagiellonian University, Krakow,Polandf; National Institute of Laser Enhanced Science (NILES),Cairo University, Cairo,Egypt9; Harvard-MIT Division of Health Sciences and Technology, Cambridge,Massachusetts, USAh ABSTRACTRRose bengal (RB) is a halogenated xanthene dye that has been used tomediate antimicrobial photodynamic inactivation for several years. While RB is highlyactive against Gram-positive bacteria, it is largely inactive in killing Gram-negativebacteria. We have discovered that addition of the nontoxic salt potassium iodide(100 mM) potentiates green light (540-nm)-mediated killing by up to 6 extra logswith the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa, theGram-positive bacterium methicillin-resistant Staphylococcus aureus, and the fungalyeast Candida albicans. The mechanism is proposed to be singlet oxygen additionto iodide anion to form peroxyiodide, which decomposes into radicals and, finally,forms hydrogen peroxide and molecular iodine. The effects of these different bacte-ricidal species can be teased apart by comparing the levels of killing achieved inthree different scenarios: (i) cells, RB, and Kl are mixed together and then illumi-nated with green light; (ii) cells and RB are centrifuged, and then Kl is added andthe mixture is illuminated with green light; and (iii) RB and Kl are illuminated withgreen light, and then cells are added after illumination with the light. We alsoshowed that Kl could potentiate RB photodynamic therapy in a mouse model ofskin abrasions infected with bioluminescent P. aeruginosa. KEYWORDS antimicrobial photodynamic inactivation, rose bengal, Pseudomonasaeruginosa mouse infection, potassium iodide, reactive iodine species, singlet oxygen he rapid and seemingly unstoppable rise in the incidence of antibiotic resistancenecessitates the development of alternative antimicrobial strategies (1). Antimicro-bial photodynamic inactivation (aPDI) employs the combination of nontoxic dyes calledphotosensitizers (PS), which can be excited by harmless visible light (2). In the presenceof ambient oxygen, the excited-state PS undergoes a photochemical reaction, produc-ing reactive oxygen species (ROS), such as singlet oxygen (10,) and hydroxyl radicals(HO"). These highly reactive species are able to chemically attack most biomolecules,such as proteins, nucleic acids, and lipids. In principle, the damage produced by thischemical attack can kill any known type of microorganism, including Gram-positive andGram-negative bacteria, fungi, parasites, and viruses. However, having said that, it mustbe noted that there is a major difference between Gram-positive and Gram-negativebacteria (3). This difference arises because of the different structural characteristics of Received 3 March 2017 Returned formodification 18 March 2017 Accepted 19April 2017 Accepted manuscript posted online 24April 2017 Citation Wen X, Zhang X, Szewczyk G, El-Hussein A, Huang Y-Y, Sarna T, Hamblin MR.2017. Potassium iodide potentiatesantimicrobial photodynamic inactivationmediated by rose bengal in in vitro and in vivostudies. Antimicrob Agents Chemother61:e00467-17.https://doi.org/10.1128/AAC.00467-17. Hamblin,hamblin@helix.mgh.harvard.edu. 25 FIG 1 Effect of KI on light dose-dependent aPDI. Cells were incubated for 30 min with RB (at the specified concentration) plus 100 mM Kl, and then 540-nmlight was delivered (0, 10, or 20 J/cm2). Controls were cells alone or cells incubated with KI alone. (A) Gram-negative bacterium E. coli; (B) Gram-positivebacterium MRSA; (C) fungal yeast C. albicans. the cell walls of these two classes of bacterial cells. Gram-positive bacterial cell walls arehighly permeable, and most dyes can diffuse easily into the cells (hence the namereferring to the Gram stain). On the other hand, Gram-negative bacteria are mostlyimpermeable to many dyes that have anionic or neutral charges. Only dyes withpronounced cationic charges can bind and penetrate into Gram-negative bacterial cellsbecause the double membrane presents a formidable barrier against penetration ofmost PS that have been developed for anticancer applications (4). One dye that has been frequently studied for antimicrobial PDI applications isthe halogenated xanthene known as rose bengal (RB)(5-9). RB (4,5,6,7-tetrachloro-2',4',5',7'-tetraiodofluorescein) is a deeply colored pink compound with a variety ofmedical uses. It is used as a stain to identify corneal damage (10), in the diagnosis ofbrucellosis (11), and as a PS to mediate photochemical tissue bonding (12). It has alsobeen used as an anticancer compound (in the dark) given as an intralesional injectioninto transit melanoma metastases, where it has been shown to activate an antitumorimmune response (13). When used as an antimicrobial PS, RB has been found to havea major effect against Gram-positive bacteria and is also effective against fungi, such asCandida spp., but its activity against Gram-negative bacteria is minimal. We have recently found that the nontoxic inorganic salt potassium iodide has aremarkable potentiating effect on aPDI (14). Addition of a simple Kl solution to amixture of microbial cells and many different PS (phenothiazinium dyes [15, 16],titanium dioxide photocatalysis [17], and fullerenes [18]) that are subsequently excitedby light can give many log units of additional killing (up to 6 logs of extra killing). Werecently showed (19) that addition of KI to the traditional anticancer, porphyrin-basedPS called Photofrin (PF; porfimer sodium) allowed broad-spectrum aPDI, including theeradication of Gram-negative bacteria that were previously thought to be resistant toPF photodynamic therapy (PDT) (17). In the present study, we asked the questionwhether the aPDI effects of the anionic dye RB would also be potentiated by additionof KI. RESULTS Our initial experiments involved comparison of the microbial killing achieved withincreasing light doses when KI was added into the mixture of cells incubated with theappropriate concentration of RB and then illuminated with green light. Because it isknown that Gram-positive bacteria are very susceptible to aPDI with anionic PS, such asRB, we needed to use only a very low concentration of 100 nM.For Escherichia coli andCandida albicans, we used a higher concentration (but not excessively high) of 10 uM.We used a100 mM concentration of KI. The results are displayed in Fig. 1. At the lowestlight dose tested (10 J/cm2), RB plus Kl and light eradicated (>6logs of killing) E. coli,while only less than 1 log of killing was found when KI was omitted (Fig. 1A). Even with 20 J/cm2, RB plus light gave only 2 logs of killing. Methicillin-resistant Staphylococcusaureus (MRSA) was killed 1 to 2 logs by the low concentration (100 nM) of RB plus light,while the addition of KI gave 4 logs of additional killing at 10 J/cm² and eradication at20 J/cm2 (Fig. 1B). For C. albicans, the potentiation was even more dramatic (Fig. 1C).The lowest light dose gave eradication when KI was present, while no killing was foundwith RB plus light at either fluence. ln order to distinguish between the photochemical oxidation of iodide to givemolecular iodine, which is a stable molecule that can exert an antimicrobial effect, andthe photochemical creation of reactive iodine species, which would be short-lived andwould exert an antimicrobial effect only when the cells were present as the light wasdelivered, we carried out the following series of experiments. We increased theconcentrations of Kl, as it became apparent that surprisingly high concentrations werenecessary to obtain the maximal potentiation effect. We reasoned that if more killingwas observed when the cells were present during the illumination than when the cellswere added after the illumination, then it could be deduced that some short-livedspecies as well as the stable molecular iodine were involved in the killing. In order tostudy to what extent the RB that was bound to the microbial cells (as opposed to theRB free in solution) was involved in the KI potentiation of aPDI, we also added a stepinvolving the centrifugation of the cells after incubation with RB. The results are shownin Fig. 2. Figure 2A and B show the results for the Gram-negative species E. coli andPseudomonas aeruginosa treated with 10 uM RB and 10 J/cm² of 540-nm light. Theresults are quite similar. When all the ingredients were present at the same time (Fig.2A and B, closed squares), there was modest killing (1 to 2 logs) at up to 10 mM Kl, butat 25 mM KI there was eradication (7 logs of killing). When the cells were added onlyafter the Kl and RB had been illuminated, there was no major killing until the KIconcentration reached 50 mM (when there was 3 to 4 logs of killing), and at 100 mMKl there was eradication (Fig. 2A and B, open circles). When the cells that had beenincubated with RB were centrifuged, there was virtually no killing even with 100 mM KI.This result is consistent with a lack of binding between RB and Gram-negative bacteria. Figure 2C shows the results obtained with the Gram-positive bacterium MRSA.Because MRSA is exceptionally sensitive to RB-mediated aPDI, we used the very lowconcentration of 100 nM RB. aPDI with a very low concentration of KI (aPDI equivalentto that with RB alone) gave 1 log of killing, and at higher concentrations (up to 25 mMKl) there were 2 logs of killing. However, at 50 and 100 mM Kl, there were 5 to 6 logsof killing. When the cells were added after the light, we found only 1 to 2 logs of killingeven with 100 mM KI. There was no killing after a spin. Figure 2D shows the results obtained with C. albicans using 10 uM RB. With allingredients present (closed squares) and with no Kl, we obtained 1.5 logs of killing, andthis remained the same until a Kl concentration of 10 mM was reached. We began tosee potentiation at 25 mM Kl, and this increased until eradication was achieved with100 mM Kl. When the cells were added after light (open circles), we saw only minimalkilling even at 100 mM KI. When the cells were centrifuged after incubation with RB (Fig.2D, open squares), we saw about 1 log of killing, and this did not really increase withan increase in the KI concentration up to 100 mM. Since the results of the experiments where the cells were centrifuged after incuba-tion with RB suggested that only C. albicans cells actually bound any RB, we carried outconfocal microscopy imaging studies to look at the RB fluorescence in cells that hadbeen centrifuged to confirm these findings. Figure 3A shows that C. albicans had adistinct green fluorescence emission around the cells, but the fluorescence did notpenetrate to any great extent inside the cells. Mechanistic studies. We carried out a range of experiments to elucidate themechanism of action of the potentiation of RB-mediated aPDI by Kl. Initially, weconfirmed that free iodine was generated in a light dose-dependent manner by usingthe well-known formation of a blue inclusion complex with soluble starch (Fig. 4A).Next,we confirmed the generation of hydrogen peroxide using the Amplex red assay FIG 2 Effect of spin and addition sequence on RB plus KI aPDI. RB (10 uM for Gram-negative bacteria and fungi or 100 nM for MRSA) wasexposed to 10 J/cm² of 540-nm light in the presence of different concentrations of KI. Cells (108/ml for bacteria or 107/ml for fungi) wereeither present during light, centrifuged before addition of KI and light, or added after light. Controls (light alone or light plus KI) showedno loss of viability (data not shown). (A) Gram-negative bacterium E. coli; (B) Gram-negative bacterium P. aeruginosa;(C) Gram-positivebacterium MRSA; (D) fungal yeast C. albicans. (Fig.4B), as we had previously observed that H02 was generated by Photofrin and KIexcited by blue light (19). We reasoned that there were two possible routes by whichhydrogen peroxide could be formed. One involves a one-electron transfer from iodideto singlet oxygen to produce superoxide, which could then undergo dismutation togive H20. The other route involves addition of singlet oxygen to iodide anion to giveperoxyiodide, which would decompose to give H202 and iodine. To distinguish be-tween these two possible routes, we used the nitroblue tetrazolium (NBT) assay forsuperoxide (20), reasoning that if we detected superoxide, the first route was sug-gested, while if we did not detect it, then the second route may take place.In order tobe sure that our failure to detect superoxide was real, we needed a positive control, andthis was obtained by illuminating a water-soluble fullerene with UV-A light in thepresence of NADH (21, 22). Figure 4C shows that there was no detectable superoxideproduced by RB plus Kl and 540-nm light, while we detected superoxide from themonocationic fullerene BB4 plus NADH and 360-nm light. In order to confirm that singlet oxygen, as opposed to some type I ROS, was theprincipal mediator of the potentiated microbial killing, we asked whether Kl could FIG 3 Confocal microscopy. Confocal images of C. albicans incubated with RB (10 uM) for 30 min and centrifuged. (A) Emission at 585nm; (B) grayscale. quench the activation of the fluorescent probe for 102 called "singlet oxygen sensorgreen" (SOSG) when RB was excited by 540-nm light. Figure 5A shows a significantquenching of SOSG activation by addition of 100 mM KI. KI quenching of the singletoxygen photogenerated by RB was also demonstrated by direct measurement of thesinglet oxygen lifetime. Figure 5B shows representative time-resolved kinetics of theformation and decay of the 1,270-nm phosphorescence detected in a control samplewithout Kl and in the presence of 35 mM KI. At the concentration used, KI shortenedthe observable lifetime of the singlet oxygen-dependent luminescence more than3-fold. It is also apparent that the initial intensity of the singlet oxygen phosphores-cence was reduced in the sample containing Kl. The observable lifetime of singletoxygen and its initial intensity as a function of KI concentration are shown in Fig. 5C.The bimolecular rate constant of quenching (k) of singlet oxygen by Kl, derived fromthe plot, was 1.1 × 106 M-1s-1. The chemical nature of singlet oxygen quenching byKI is clearly demonstrated by oxygen consumption measurements. Figure 5D showsthat while in the absence of KI there was no measurable oxygen consumption, addition FIG 4 Mechanistic experiments. (A) Production of iodine (measured as blue starch complex) by RB + KI+ green light; (B) production of hydrogen peroxide(measured by Amplex Red assay) by RB + KI+ green light; (C) lack of production of superoxide (measured by nitroblue tetrazolium assay) by RB + KI + greenlight; fullerene + NADH + 360-nm light was used as positive (+ve) control.AU, absorbance units. A wco FIG 5 Mechanistic experiments. (A) Activation of SOSG by RB (100 nM) excited by 540-nm light with and without added KI (100 mM); (B) time-resolved kineticsof the formation and decay of 1,270-nm phosphorescence detected in the presence and absence of 35 mM KI; (C) lifetime of singlet oxygen and its initialintensity as a function of the Kl concentration; (D) oxygen consumption by illuminated RB in the presence and absence of 2 concentrations of Kl. of Kl induced the depletion of oxygen, with the initial rate being dependent on the KIconcentration. In vivo studies.We used a mouse model of a partial-thickness skin wound (abra-sion) to test the novel combination (RB plus KI and 540-nm light) in vivo. We chose P.aeruginosa as the bacterial pathogen for the following reasons: (i) P. aeruginosa is aGram-negative bacterial species and would not be expected to be much affected by RBplus light alone (without KI), (ii) P. aeruginosa is sufficiently pathogenic to form along-lasting infection with a reasonable infective dose of cells, and (iii) the particularstrain of P. aeruginosa that we used is not sufficiently virulent in this model to cause asystemic infection, which would lead to death of the mice. When we inoculated 5× 105 CFU of bioluminescent P. aeruginosa (in 50 ul) into thepartial-thickness abrasion wound on the back of the mice, a stable infection that lastedfor longer than 6 days was established (Fig. 6). Figure 7 shows a panel of biolumines-cence images from representative mice in each of the four groups captured before light(0 J/cm2) and after 10 J/cm² and 20 J/cm² of 540-nm light had been delivered. Theno-treatment control and RB plus KI dark groups (Fig. 7, rows 1 and 2) did not show anyreduction in the bioluminescence signal, while the RB plus 540-nm light groups showedonly a slight reduction. In contrast, the group treated with RB plus Kl and 540-nm lightshowed a major reduction after 10 J/cm²was delivered, and after 20 J/cm² wasdelivered, the bioluminescence signal was undetectable. Figure 8A shows the quanti-fication of the signals from 5 mice per group. Figure 6 shows the monitoring ofrepresentative mice from four groups for six successive days after PDT. It can be seen Luminescence 06 max=1.8e6 FIG 6 Monitoring of aPDT of skin abrasions infected with P. aeruginosa in the days following light delivery. Images fromrepresentative mice from the four groups for which the results are shown Fig. 5 were monitored for bioluminescence eachday from day 0 (before PDT) until day 6. no Tx, no treatment. FIG 7 Monitoring of aPDT of skin abrasions infected with P. aeruginosa during light delivery. Represen-tative bioluminescence images from a mouse from each of the treatment groups are shown: theno-treatment control group, the group treated with 50 ul of RB(500 uM) plus KI (1 M) in the dark, thegroup treated with 50 ul of RB (500 uM) plus PDT, and the group treated with 50 ul of RB (500 uM) plusKI (1 M) and PDT. Images were captured after delivery of 0, 10, or 20 J/cm² of 540-nm light (rows 3 and4) or after equivalent times had elapsed (rows 1 and 2). that there is not much difference between the groups. The only noticeable observationis that the signal from the RB plus KI and light group was significantly lower than thatfrom each of the other three groups (P<0.05) on day 1 (i.e., the day immediatelyfollowing PDT). Figure 8B shows the quantification of the bioluminescence signals. Theregrowth of the luminescence signal in the wound following the apparently successful FlG 8 Quantification of the bioluminescence signals from the mice in the groups for which the results are shown in Fig.5 and7. Points are means for 5 mice, and bars are SDs. *,P < 0.05 versus the control by one-way ANOVA. FIG 9 Histology images. (A and E) No-treatment control; (B and F) RB plus KI in the dark; (C and G) RB plus light; (D and H) RB plus Kl and light. (A to D) Gramstain; (E to H) H&E stain. Bar, 100 um. eradication by PDT has been observed before (23, 24) and is the chief drawback ofusing PDT as an antibacterial therapy in vivo. Figure 9 shows the histology images taken from mice that were sacrificed 24 h afterPDT. We stained the sections with Gram stain to visualize the P. aeruginosa cells in thetissue (Fig. 9A to D) and also with hematoxylin and eosin (H&E) (Fig. 9E to H) tovisualize any gross damage that may have been caused by the PDT or by the iodineproduced by PDT plus KI. There were clearly fewer bacteria present in the group treatedwith PDT plus KI (Fig.9D) than in the other groups (Fig. 9A to C). While H&E stainingis not necessarily the most sensitive method to detect PDT-induced tissue damage,there were no obvious signs of extra damage in Fig. 9H compared to Fig. 9E to G. DISCUSSION We have shown that addition of the simple nontoxic inorganic salt potassium iodidecan dramatically potentiate aPDI mediated by RB, especially against Gram-negativebacteria. In the case of two Gram-negative bacterial species, E. coli and the hard-to-killspecies P. aeruginosa, addition of 25 mM KI gave 7 logs of killing, whereas almost nokilling was obtained with RB aPDI in the absence of KI. In the case of the fungal yeastC. albicans, addition of 100 mM Kl gave eradication (>6 logs of killing), whereas justover 1 log of killing was achieved without KI. The data suggest that two kinds ofantimicrobial species are produced by the PDT-induced oxidation of iodide.The mostobvious antimicrobial species is molecular iodine (or triiodide in the presence ofiodide), as shown by the generation of the blue inclusion complex when starch wasadded to the reaction product obtained when 540-nm light was delivered to a mixtureof RB plus KI. Nevertheless, there is clearly another short-lived antimicrobial species thatis generated and can produce killing only when the cells are present during theillumination. With Gram-negative bacteria, eradication was achieved with 25 mM KIwhen cells were present, while 100 mM KI was necessary when the bacteria were addedafter light. In the case of C. albicans, eradication was achieved with 100 mM KI whencells were present, while hardly any killing was seen when cells were added after light.In the case of methicillin-resistant Staphylococcus aureus (MRSA) (with a low concen-tration of RB and 100 mM Kl), eradication was achieved with the cells were presentand only 1 log of killing was achieved when cells were added after light. Theexplanation for the difference in susceptibility between Gram-negative species, onthe one hand, and Gram-positive species and Candida, on the other hand, whencells were added after light probably lies in features such as the thickness of the cellwall (25). The thin cell wall typical of Gram-negative bacteria may allow iodinespecies to penetrate and kill them with an ease greater than that found with othermicrobial cells with thicker cell walls. RB does not bind well to most classes of microbial cells. This lack of binding is shownby the fact that centrifugation of the cells after incubation with RB removes most,if not all, of the light-mediated killing. Even with the Gram-positive bacteriumMRSA, against which RB is extremely active as an antimicrobial PS (since the lowconcentration of 200 nM produced eradication), centrifugation abolished the kill-ing. In the case of C. albicans, binding appears to be of some importance, sincethere was some minor killing after centrifugation; there was also potentiation with100 mM KI when cells were present but no killing when cells were added after light.As seen in Fig. 3, C. albicans was the only cell type to show any detectablefluorescence after incubation with RB. We assume that the reactive iodine speciesare more efficient in killing microbial cells when they are generated close to thecells, as might be expected with loose binding of the RB to the cell surface. If thebinding between the cells and the RB was loose, it might be expected that the RBcould be dislodged after centrifugation. RB has been found to operate largely via the type ll photochemical pathwayinvolving energy (hv) transfer from the long-lived RB triplet state to the ground-statetriplet oxygen to produce the reactive singlet oxygen (equations 1 and 2) (26). Thenumber of heavy halogen atoms (4iodine and 4 chlorine atoms) means that RB has asinglet oxygen quantum yield of about 0.86 (27). In fact, RB is often used as a standardin determinations of singlet oxygen quantum yields (28) There two possible pathways by which singlet oxygen could, in principle, react withiodide anion. The first pathway is a one-electron transfer from iodide to 10, to givesuperoxide anion and iodide radical (equation 3). The iodide radicals dimerize to give molecular iodine, which reacts with iodide to givethe triiodide anion (equation 4). lodine radicals would then be the short-lived reactivespecies that give potentiation of aPDI killing. The superoxide anion then undergoes dismutation to give hydrogen peroxide (equa-tion 5). This pathway was our initial hypothesis, after we demonstrated the formation ofboth iodine/triiodide and hydrogen peroxide, which was consistent with the proposedmechanism. However, despite numerous attempts, we were unable to show anyproduction of superoxide using the NBT assay, even though we were able to obtain apositive result with another established photochemical method to generate superox-ide. This was done by illumination of a water-soluble fullerene in the presence ofreduced NADH (21,22).Hence, we concluded that a one-electron transfer reaction fromiodide to singlet oxygen to give superoxide and the iodine radical probably did notoccur. It must also be stressed that such a reaction is thermodynamically unlikely, forit would be accompanied by an unfavorable change in free energy. This is because theone-electron reduction potentials of the corresponding couples,10,/0andI/l-, are.+0.65 V and +1.270 to 1.400 V, respectively (29). However, there is a second possible pathway between singlet oxygen and iodide.This takes the form of an addition reaction between singlet oxygen and iodide to giveperoxyiodide (equation 6). The decomposition of peroxyiodide is proposed to proceed via equations 7 to 10. Wen et al. Dalmazio et al. (30) from Brazil used mass spectrometry and ab initio free energycalculations to study the decomposition of hydrogen peroxide in the presence of iodideanions. They detected a species with m/z 287 that was proposed to be HOOl-.Calculations revealed that the thermodynamically preferred decomposition pathwaywas equation 11 to produce two radicals. Competing decomposition pathways were energetically less favored by between 25and 68 kcal/mol. These two radicals, land HOO, would account for the short-lived reactive speciesresponsible for the extra killing observed when the cells were present during theillumination. We originally discovered that the action of KI (maximum concentration, 10 mM)potentiates the aPDI mediated by the phenothiazinium salt methylene blue (MB) (16).We had previously shown that the potentiation of aPDI mediated by MB was paradox-ically potentiated by 10 mM sodium azide (singlet oxygen quencher) operating by aone-electron transfer from azide anion to excited-state MB to form azide radicals in anoxygen-independent process (31). Therefore, we assumed that the mechanism in thecase of KI and MB was an analogous one-electron transfer from the iodide anion to theexcited-state PS to form an iodine radical and an MB radical anion (16). We then wenton to show that the photocatalysis mediated by titanium dioxide nanoparticles excitedby UV-A light could also be strongly potentiated by addition of KI (18). Here themechanism was via a mixture of a one-electron oxidation of iodide anion to formmolecular iodine and a two-electron oxidation of iodide anion to form hypoiodite. Itwas not until we discovered (19) that aPDI mediated by the porphyrin PS known asPhotofrin was also able to be strongly potentiated by KI (provided the concentration ofiodide was at least 25 mM and, preferably, 100 mM) that we realized singlet oxygen waslikely to be involved in the process (19, 32). We were able to show not only thatactivation of SOSG was quenched by Kl but also that the luminescence signal of singletoxygen was quenched by iodide and that oxygen was consumed in irradiated samplescontaining RB and KI. Quenching of the characteristic singlet oxygen phosphorescenceby KI suggests that the effect is due to the interaction of KI with singlet oxygen, whichshortens the observable lifetime of singlet oxygen, and to the interaction of KI with theRB triplet excited state, which reduces the observable intensity of singlet oxygen. Whilethe former interaction is mostly chemical in nature, leading to the formation of theunstableperoxyiodide, the latter interaction could be a physical quenching of theRB triplet excited state with no specific product formed or a charge-coupled tripletdeactivation, in which the triplet excited state of RB is reduced by KI (33). AlthoughDenofrio et al. (34) reported on a very efficient quenching of both the singlet and tripletexcited states of pteridines by Kl, with the corresponding rate constants being close tothe diffusion-controlled limit, the effect observed in the present study (Fig.5A) suggeststhat the efficiency with which KI quenches the RB excited triplet state is significantlylower. This is probably due to the strong electrostatic repulsive interaction of the twomolecules, which in water at neutral pH are negatively charged (35), and to therelatively low energy level of the RB triplet excited state that makes the charge-coupledquenching mechanism inefficient. ln the present study, we were able to show the effectiveness of KI as an enhancerof RB-mediated PDT in a mouse model of a partial-thickness wound infection causedby the stubborn and drug-resistant Gram-negative bacterial pathogen P. aerugi-nosa. Although MRSA is the most problematic cause of complicated skin and softtissue infections (SSTIs), P. aeruginosa comes in second in this regard (36). Moreover, P.aeruginosa displays intrinsic antibiotic resistance, has the capacity to acquire further resistance mechanisms, and readily forms a protective biofilm in vivo (37). Amongseveral studies of aPDI, P. aeruginosa is considered to be one of the hardest bacterialspecies to kill by aPDI (38). The monitoring of the bioluminescence signal during lightdelivery did show a strong potentiation of the bactericidal effect by addition of Kl, asmight be expected from the in vitro data. Moreover, the monitoring of the biolumi-nescence signal in the days following PDT appeared to show that the addition of KI alsoappeared to give some benefit in inhibiting recurrence, especially on the day after PDT.lt has become apparent that the main drawback to using PDT as an antibacterial interven-tion in models of localized infection is the fact that after the light has been turned off, thegeneration of antimicrobial species ceases and any remaining bacteria are completely freeto regrow. However, in the present case of added Kl, it is likely that free iodine/triiodide isgenerated within the wound by the action of photogenerated singlet oxygen on iodideanions. This free iodine/triiodide may remain active within the wound for a much longertime and may inhibit bacterial regrowth for some time to come. We believe that the action of KI to potentiate aPDT is sufficiently impressive andthat, in conjunction with its nontoxic nature, it could progress into clinical testing forthose infections where aPDT is being clinically explored, such as periodontitis orchronic sinusitis (39). MATERIALS AND METHODS Chemicals and reagents. Rose bengal (RB), potassium iodide (KI), nitroblue tetrazolium (NBT), andall other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.An Amplex red hydrogen peroxide/peroxidase assay kit was purchased from Invitrogen (Carlsbad, CA,USA). The singlet oxygen sensor green (SOSG) and hydroxyphenyl fluorescein (HPF) probes used todetect singlet oxygen or hydroxyl radicals were purchased from Life Technologies (Grand Island, NY,USA); the starch indicator was purchased from Ricca Chemical Company (Arlington, TX, USA). RB stocksolution (5 mM) was prepared in distilled H,0 (dH,O) and was stored at 4℃ in the dark for no more than2 weeks prior to use. KI solution was prepared in dH,O as required immediately before experimentation.Light source. A green light source consisting of a white lamp with a band-pass filter probe(wavelength, 540 ± 15 nm; Lumacare, Newport Beach,CA, USA) was used to deliver light over a spot of4 cm in diameter that covered four wells of a 24-well plate in vitro or the whole abrasion in vivo at anirradiance of 100 mW/cm2. Light power was measured with a power meter (model DMM 199 with a 201 standard head; Coherent, Santa Clara, CA, USA). Cells and culture condition. The following microbial strains were used: the Gram-positive bacteriummethicillin-resistant Staphylococcus aureus (MRSA) USA 300, Gram-negative bacteria Escherichia coli K-12(ATCC 33780) and Pseudomonas aeruginosa ATCC 19660 (Xen 5P), and strain CEC 749 of the luciferase-expressing fungal yeast Candida albicans. A colony of bacteria or fungal yeast was suspended in 20 ml of brainheart infusion (BHI) broth for bacteria or yeast extract-peptone-dextrose (YPD) for C. albicans and grownovernight in a shaker incubator (New Brunswick Scientific, Edison, NJ) at 120 rpm under aerobic conditionsat 37℃ for bacteria and at 30℃ for C. albicans. For bacteria, an aliquot of 1 ml from an overnight bacterialsuspension was refreshed in fresh BHI for 2 to 3 h at 37℃ to mid-log phase. The cell concentration wasestimated by measuring the optical density (OD) at 600 nm (OD of 0.6 = 108 cells/ml). The C. albicans cellnumber was assessed with a hemocytometer and was generally between 107 and 108 cells/ml. In vitro studies. Two types of in vitro aPDI experiments were done. The first used cells with a fixedRB concentration and KI concentration and varied the light dose. The second type of experiments usedcells with a fixed RB concentration and a fixed light dose and varied the Kl concentration. Here wecompared the order of addition of the components and the effect of centrifugation. The initial studiesused PDI, with suspensions of bacteria (108 cells/ml) or C. albicans (107 cells/ml) being irradiated withdifferent fluences of green light (0, 10, and 20 J/cm2) and with different concentration of RB (100 nM forMRSA, 10 uM for E. coli and C. albicans) with or without 100 mM KI. The aliquots were serially diluted10-fold in phosphate-buffered saline (PBS) to give dilutions of 10-1 to 10-5, in addition to the originalconcentration, and 10-ul aliquots of each of the dilutions were streaked horizontally on square BHI agarplates for bacteria or YPD agar plates for Candida. Plates were streaked in triplicate and incubated for 12to 18 h at 37℃(bacteria) or for 24 to 36 h at 30℃ (Candida) in the dark to allow colony formation.Eachexperiment was performed at least three times. Suspensions of bacteria (108 cells/ml) or C. albicans (107cells/ml) were incubated in the dark at roomtemperature for 30 min with 10 uM RB (for Gram-negative bacteria and C. albicans) or 100 nM RB (forMRSA), and then Kl at concentrations ranging from 0 to 100 mM in PBS (pH 7.4) was added. Centrifu-gation (5 min, 3,200 rpm) of 1-ml aliquots was used to remove the excess of RB that was not taken upby the microbial cells when experiments required it. The 1-ml aliquots were transferred to a 24-well plate, and the tops of the plates were illuminated with10 J/cm2 of green light in the dark at room temperature. Care was taken to ensure that the contents ofthe wells were mixed thoroughly before sampling, as bacteria can settle at the bottom. The aliquots wereserially diluted as described above. When experiments required it, 10 uM RB or 100 nM RB plus Kl at concentrations ranging from 0 to100 nM in PBS (pH 7.4) was exposed to 10-J/cm2 green light, and then bacterial or C. albicans cells wereadded to the illuminated mixture of RB plus Kl. After 30 min of incubation, the aliquots were seriallydiluted as described above. Each experiment was performed at least three times. RB (10 uM or 100 nM) and KI at a range of concentrations were exposed to 10 J/cm2 of green light,and then 108 cells/ml of bacteria or 107 cells/ml of C. albicans were added and the mixture was incubatedin the dark at room temperature for 30 min. The aliquots were serially 10-fold diluted as described above.Each experiment was performed at least three independent times. A control group of cells treated with light alone (no RB added) showed the same number of CFU asthe absolute control (data not shown). Survival fractions were routinely expressed as ratios of the numberof CFU of microbial cells treated with light and RB (or RB in the absence of light) to the number of CFUof microbes treated with neither. Confocal scanning laser microscopy. Suspensions of E. coli or MRSA (108 cells/ml) or of C. albicans(107 cells/ml) were incubated in the dark at room temperature for 30 min with 10 uM RB, and then 1-mlaliquots were centrifuged (3 min, 3,200 rpm) to remove the excess of RB. A confocal scanningfluorescence microscope (Olympus American Inc., Melville, NY) was employed to observe the fluores-cence emission of RB and cells. Fluorescent images were obtained with a 585-nm band-pass filter.Excitation was at 543 nm. Mechanistic experiments. (i) lodine starch test. RB (10 uM) and KI (100mM) were illuminated withincreasing fluences of green light, and aliquots (50 ul) were withdrawn after illumination at the differentfluences and added to the starch indicator (50 ul). A microplate reader (absorbance at 610 nm) was usedto measure the incremental absorbance after an incremental fluence of 415 nm of light was delivered.Controls were (i) RB plus light, (ii) Kl plus light, and (iii) PBS alone. Each experiment was performed at leastthree times. (ii) Amplex red assay for hydrogen peroxide. An Amplex red hydrogen peroxide/peroxidase assaykit was used to detect the production of H,O,from RB-and Kl-mediated PDT. The colorless probe Amplexred (10-acetyl-3,7-dihydroxy-phenoxazine) reacts with H202 in the presence of peroxidase and formsresorufin (7-hydroxy-3H-phenoxazin-3-one). The detection process after RB- and Kl-mediated PDT wasdone according to the manufacturer's instructions. The reaction systems contained 2 uM RB with added50 mM Kl and were illuminated with increasing fluences of green light, and aliquots were withdrawnand added to 50 uM Amplex red reagent and 0.1 U/ml horseradish peroxidase (HRP) in Krebs-Ringerphosphate (which consists of 145 mM NaCl, 5.7 mM Na,PO, 4.86 mM KCl, 0.54 mM CaCl,, 1.22 mMMgSO, 5.5 mM glucose, pH 7.35). After 30 min of incubation, a fluorescence microplate reader(excitation, 530 nm; emission,~590 nm) was used to measure the incremental fluorescence after anincremental fluence of green light was delivered. Controls were (i) RB plus light, (ii) Kl plus light, and (iii)Amplex red reagent alone. Each experiment was performed at least three times. (iii) NBT. The superoxide assay employed NBT at 20 mM, RB at 10 uM, and Kl at 50 mM, and all threewere dissolved in PBS. All ingredients were freshly prepared prior to the procedure; an absorbancemicroplate reader was used to measure the incremental absorbance of the blue product (560 nm) afteran incremental fluence of green light was delivered. Controls were (i) RB plus light, (ii) Kl plus light, and(iii) PBS alone. Each experiment was performed at least three times. A monocationic fullerene, BB4 (35uM in combination with 1 mM NADH) (21), excited by 360-nm light was used as a positive control forphotogenerated superoxide production. (iv) Activation of SOSG. Cell-free experiments were performed in 96-well plates.RB was used at100 nM in PBS, and SOSG (Molecular Probes, Invitrogen, USA) was added to each well at a finalconcentration of 5.0 uM. KI solution (100 mM KI) was either added or not added. Each experimentalgroup contained four wells. All groups were illuminated simultaneously, and light was delivered insequential doses of 1.0 to 4 J/cm2. A microplate spectrophotometer (Spectra Max M5; MolecularDevices) was used for the acquisition of fluorescence signals in the slow kinetic mode. Thefluorescence excitation was at 505 nm and emission was at 525 nm. Each time after an incrementalfluence was delivered, the fluorescence was measured. (v) Interaction of singlet oxygen with iodide. The interaction of iodide with singlet oxygenphotogenerated by RB was examined directly by measuring the lifetime of singlet oxygen at differentconcentrations of KI and indirectly by monitoring the effect of increasing concentrations of iodide onoxygen uptake induced by irradiation of the RB solution with green light (40). In brief, time-resolved singlet oxygen detection was carried out as follows. Phosphate-buffered (pH7.2) D,0 solutions of RB (optical density, ~0.25 to 0.3 at 550 nm) in a 1-cm-optical-path quartzfluorescence cuvette (catalog number QA-1000; Hellma, Mullheim, Germany) were excited by 550-nmpulses generated by an integrated nanosecond neodymium-doped yttrium aluminum garnet lasersystem equipped with a narrow-band-width optical parametric oscillator (model NT242-1k-SH/SFG; Ekspla,Vilnius, Lithuania), which delivered pulses at a repetition rate of 1 kHz with energy of up to several hundredmicrojoules in the visible region. Due to the high efficiency of singlet oxygen photogeneration by RB, theenergy of the exciting pulses was attenuated ~400 times. The near-infrared luminescence (1,270 nm) wasmeasured perpendicularly to the excitation beam in a photon-counting mode using a thermoelectric coolednear-infrared photomultiplier tube (NIR PMT) module (model H10330-45; Hamamatsu, Japan) equipped witha 1,100-nm-cutoff filter and an additional dichroic narrow-band filter narrow-band pass (NBP), selectable fromthe spectral range of 1,150 to 1,355 nm (NDC Infrared Engineering Ltd., Maldon, Essex, UK). Data werecollected using a computer-mounted peripheral component interconnect (PCI)-board multichannel scaler(model NanoHarp 250; PicoQuant GmbH, Berlin, Germany). Data analysis, including first-order luminescencedecay fitted by the Levenberg-Marquardt algorithm, was performed by custom-written software. A typical acquisition time was 20 s. The effect of potassium iodide on the singlet oxygen lifetime was examined overa concentration range of from 0 to 50 mM. (vi) Oxygen photoconsumption measurements.Time-dependent changes in the oxygen concen-tration induced by light were determined by electron paramagnetic resonance (EPR) oximetry using 0.1mM 4-hydro-3-carbamoyl-2,2,5,5-tetramethyl-pyrrolin-1-oxyl (mHCTPO) as a dissolved-oxygen-sensitivespin probe. Samples containing 25 uM RB in PBS, pH 7.2, were irradiated in EPR quartz flat cells in theresonant cavity with 516- to 586-nm(35-mW/cm2) light derived from a 300-W high-pressure compact arcxenon lamp (Cermax; model PE300CE-13FM/Module300W; PerkinElmer Opto-Electronics, GmbH, Wies-baden, Germany) equipped with a water filter, a heat-reflecting mirror, a cutoff filter blocking light below390 nm, and a green additive dichroic filter (catalog number 585FD62-25; Andover Corporation, Salem,NC, USA). For EPR, samples were run using microwave power of 1.06 mW, a modulation amplitude of0.006 mT, a scan width of 0.3 mT, and a scan time of 21 s. Thirty subsequent scans were acquired every30 s. EPR measurements were carried out using a Bruker EMX-AA EPR spectrometer (Bruker BioSpin,Rheinstetten, Germany). In vivo studies. (i) Bacterial strain and culture conditions. The Pseudomonas aeruginosa strainused in this work was ATCC 19660 (Xen 5P). Bacteria were routinely grown in BHI with aeration in anorbital incubator at 100 rpm and 37℃ overnight to stationary phase. An aliquot of this suspension wasthen refreshed in fresh BHI to mid-log phase. Cell numbers were estimated by measuring the OD at 600nm (OD of 0.6= 108 CFU cells/ml). The bacterial suspension was centrifuged, washed, and resuspendedin PBS, and the suspension was diluted 10-fold for the in vivo experiments. (ii) Bioluminescence imaging. An IVIS Lumina series III in vivo imaging system (PerkinElmer, Inc.,Waltham, MA, USA) was applied for bioluminescence imaging on a daily basis until the disappearance ofthe infection by bioluminescence imaging. Using the photon counting mode, an image can be obtainedby detecting and integrating individual photons emitted by the bacterial cells. Prior to PDT and imaging,mice were anesthetized by intraperitoneal (i.p.) injection of a ketamine-xylazine cocktail. Mice were thenplaced on an adjustable stage in the imaging chamber positioned directly under the camera. A grayscalebackground image of each mouse was made, and this was followed by the collection of a biolumines-cence image of the same region displayed in a false color scale ranging from red (most intense) to blue(least intense) and superimposed on the grayscale image. The signal from the bioluminescence imagewas quantified as the region of interest (ROl), with absolute calibrated data being given as the numberof photons second-1 centimeter-2 steradian-1, using the IVIS software. (iii) Pseudomonas aeruginosa infection in mice. All animal experiments were approved by theSubcommittee on Research Animal Care (IACUC) of the Massachusetts General Hospital and met NationalInstitutes of Health (NIH) guidelines. Adult female BALB/c mice that were 6 to 8 weeks old and thatweighed 18 to 21 g were used (Charles River Laboratories, MA, USA). Mice were given access to food andwater ad libitum and maintained on a 12-h light/12-h dark cycle under a room temperature of 21C. Micewere anesthetized by i.p. injection of a ketamine-xylazine cocktail. The dorsal skin of the mice was shavedwith an electric razor. To create abrasion wounds, surgical scalpels were used to gently scrape the epidermisoff an area of approximately 1 cm2. The depth of the wound was no more than the shallow dermis. Aftercreating the wounds, an aliquot of a 50-ul suspension containing 5 × 105 CFU of P. aeruginosa in PBSwas inoculated over each defined area containing the abrasion with a pipette tip. Bioluminescenceimages were taken immediately after the inoculation of bacteria to ensure that the bacterial inoculumapplied to each abrasion was consistent. (iv) In vivo PDT. Mice were divided into four groups of 5 mice each. The sample size was determinedby an 80% power to distinguish between RB PDT and RB plus KI PDT at a significance level with a P valueof 0.05 with a large effect size in relative light unit (RLU) measurements.The groups were as follows.(i)The infected control group consisted of mice whose wounds were only infected with P.aeruginosa. (ii)The dark control group consisted of mice treated with RB with KI but no light. (iii) The RB PDT groupconsisted of mice treated with RB only but irradiated with 540-nm light. (iv) The RB plus KI PDT groupconsisted of mice irradiated with 540-nm light in the presence of RB and Kl. At 30 min after application of the bacteria to the abrasions, a small aliquot of RB solution (500 uM)alone or RB (500 uM) mixed with KI (1 M) solution was added to the PDT-treated wound and also to thedark controls. Initially,50 ul of the RB solution was added to the abrasions and incubated for 10 min tobind to and penetrate the bacteria. Then, the wounds were irradiated with green light at a fluence of upto 20 J/cm², and luminescence imaging was performed after irradiation. In this case, the mice wereimaged daily to quantify the recurrence of bioluminescence until the bioluminescence disappeared orthe animals were determined to be moribund and euthanized (this did not occur with the strain of P.aeruginosa used in the present study). (v) Histological analysis. Two mice per group were used for further histological analysis. Theprocedure was the same as that described above. The mice were then sacrificed at the first day (24 h)after the experiment to compare the antibacterial effects. Removed tissue samples were fixed in asolution of 10% formalin in 100% PBS for 2 to 3 days. After fixation, samples were embedded in paraffinblocks, sectioned to a 6-um thickness, and stained with hematoxylin-eosin and also with Gram stain.Stained slides were assessed under a light microscope (Olympus BX51 microscope) to observe anyinflammation or gross tissue damage. Statistical methods. Means were calculated and compared for statistical significance using aone-way analysis of variance (ANOVA). P values of <0.05 were considered statistically significant. ACKNOWLEDGMENTS This work was supported by U.S. NIH grants R01Al050875 and R21AI121700 (toM.R.H.). X.W. was supported by the West China Hospital of Sichuan University. X.Z. wassupported by the Research Experience for Undergraduates (REU) Program of theNational Science Foundation, Award number EEC-1358296. A.E.-H. was supported bythe Fulbright Foundation. Research carried out at the Jagiellonian University (G.S. andT.S.) was supported in part by grants from the Poland National Science Center (2011/03/B/NZ1/00007 and 2013/08/W/NZ3/00700). ( 1 . O 'Neill Commission. 2015. Tac k ling a gl o b a l healt h crisis: initial ste p s. The r eview on antimicrobial r e sistance, chaired by Jim O ' Neill. B ritish Society for Antimicrobial Chemotherapy, London, United Kingdom. ) 2. Hamblin MR, Hasan T. 2004. Photodynamic therapy: a new antimicrobialapproach to infectious disease? Photochem Photobiol Sci 3:436-450.https://doi.org/10.1039/b311900a. 3. Malik Z, Ladan H, Nitzan Y. 1992. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J Photochem Pho-tobiol B 14:262-266. https://doi.org/10.1016/1011-1344(92)85104-3. ( 4. M innock A, Vernon DI, Schofield J, Griffiths J, P a rish JH, Brown SB. 2000.Mechanism of uptake of a cationic water-soluble pyridinium zinc phtha-locyanine a cross t he o uter m embrane of E sc h erichia coli. A n timicrobAgents C hemother 44:522-527. ht tps : / / d o i . o r g/10.1128/AAC.44. 3 . 5 22 -5 2 7 .2000. ) ( 5. B anks JG, Board R G , C a rter J , D odge AD. 19 8 5. The cytotoxic and pho t ody-namic inactivation of micro-organisms b y Rose B e ngal. J A p pl B a cteriol58:391-400. h t tps://doi . org/10.11 1 1/j.1365 - 2672. 1 985.tb01 4 7 8 .x. ) ( 6. Bezman S A , Burtis P A, Izod TP, Thayer MA. 1978. Photodynamic i n acti-vation of E. coli by Rose Bengal i mmobilized o n polystyrene beads.Photochem Ph o tobiol 28 : 325-329. htt p s://d oi .org/ 10. 11 1 1 / j.1 7 51-1 0 97 .1 978.tb07714.x. ) 7. Schafer M, Schmitz C, Facius R, Horneck G, Milow B, Funken KH, OrtnerJ. 2000. Systematic study of parameters influencing the action of RoseBengal with visible light on bacterial cells: comparison between thebiological effect and singlet-oxygen production. Photochem Photobiol71:514-523. https://doi.org/10.1562/0031-8655(2000)071<0514:SSOPIT>2.0.CO;2. 8. Demidova TN, Hamblin MR. 2005. Effect of cell-photosensitizer bindingand cell density on microbial photoinactivation. Antimicrob AgentsChemother 49:2329-2335. https://doi.org/10.1128/AAC.49.6.2329-2335.2005. 9. Costa AC, Rasteiro VM, Pereira CA, Rossoni RD, Junqueira JC, Jorge AO.2012. The effects of Rose Bengal- and erythrosine-mediated photody-namic therapy on Candida albicans. Mycoses 55:56-63.https://doi.org/10.1111/j.1439-0507.2011.02042.x. 10. Doughty MJ. 2013. Rose Bengal staining as an assessment of ocularsurface damage and recovery in dry eye disease-a review. Cont LensAnterior Eye 36:272-280. https://doi.org/10.1016/j.clae.2013.07.008. 11. Ducrotoy MJ, Bardosh KL. 2017. How do you get the Rose Bengal test atthe point-of-care to diagnose brucellosis in Africa? The importance of asystems approach. Acta Trop 165:33-39. https://doi.org/10.1016/j.actatropica.2016.10.004. 12. Gu C, Ni T, Verter EE, Redmond RW, Kochevar IE, Yao M. 2011. Photo-chemical tissue bonding: a potential strategy for treating limbal stemcell deficiency. Lasers Surg Med 43:433-442.https://doi.org/10.1002/lsm.21066. ( 13. Lippey J, Bousounis R, Behrenbruch C, McKay B, S pillane J, Henderson MA, Speakman D, G yo r ki DE. 2 016 . Intralesional PV-1 0 for in-transitmelanoma—a single-center e x perience. J Surg Oncol 114 : 380-384. https://doi.org/ 1 0. 1 002/jso. 2 43 1 1 . ) ( 14. Kashef N, Hamblin M R. Advances in antimicrobial p hotodynamic in a cti-vation at the nanoscale. Nanophotonics, in press. ) ( 15. Freire F , Ferraresi C, Jorge AO, Hamblin MR. 2016. Photodynamic therapy of oral Candida infection in a mouse m odel. J P hotochem Ph o tobiol B 159:161-168. h t t ps :/ / doi.org /1 0 .1 0 16/j.j ph otobi o l.2 0 16.03.049. ) ( 16. Vecchio D, Gupta A, H uang L , Landi G, Avci P, Rodas A, H amblin M R . 2015. Bacteria l photodynamic inactivation mediated by methylene blue and r ed light is enhanced by synergistic e ffect of potassium iodide. ) Antimicrob Agents Chemother 59:5203-5212. https://doi.org/10.1128/AAC.00019-15. 17. Maisch T, Baier J, Franz B, Maier M,Landthaler M, Szeimies RM, BaumlerW. 2007. The role of singlet oxygen and oxygen concentration in pho-todynamic inactivation of bacteria. Proc Natl Acad Sci U S A 104:7223-7228. https://doi.org/10.1073/pnas.0611328104. 18. Huang YY, Choi H, Kushida Y, Bhayana B, Wang Y, Hamblin MR. 2016.Broad-spectrum antimicrobial effects of photocatalysis using titaniumdioxide nanoparticles are strongly potentiated by addition of potassiumiodide. Antimicrob Agents Chemother 60:5445-5453. https://doi.org/10.1128/AAC.00980-16. 19. Huang L, Szewczyk G, Sarna T, Hamblin MR. 2017. Potassium iodidepotentiates broad-spectrum antimicrobial photodynamic inactivationusing Photofrin. ACS Infect Dis 3:320-328.https://doi.org/10.1021/acsinfecdis.7b00004. 20. Nauseef WM. 2014. Detection of superoxide anion and hydrogen per-oxide production by cellular NADPH oxidases. Biochim Biophys Acta1840:757-767. https://doi.org/10.1016/j.bbagen.2013.04.040. 21. Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, Sarna T, Hamblin MR.2007. Functionalized fullerenes mediate photodynamic killing of cancercells: type I versus type ll photochemical mechanism. Free Radic BiolMed 43:711-719. https://doi.org/10.1016/j.freeradbiomed.2007.05.005. 22. Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masum-izu T, Nagano T. 2003. Active oxygen species generated from photoex-cited fullerene (C6o) as potential medicines:02-versus 10. J Am ChemSoc 125:12803-12809. https://doi.org/10.1021/ja0355574. 23. Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, Baer DG,Hamblin MR. 2009. Photodynamic therapy for Acinetobacter baumanniiburn infections in mice.Antimicrob Agents Chemother 53:3929-3934.https://doi.org/10.1128/AAC.00027-09. 24. Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. 2010. Photo-dynamic therapy for methicillin-resistant Staphylococcus aureus infec-tion in a mouse skin abrasion model. Lasers Surg Med 42:38-44. https://doi.org/10.1002/lsm.20887. 25. Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold SpringHarb Perspect Biol 2:a000414. https://doi.org/10.1101/cshperspect.a000414. 26. Planas O, Macia N, Agut M, Nonell S, Heyne B. 2016.Distance-dependentplasmon-enhanced singlet oxygen production and emission for bacte-rial inactivation.J Am Chem Soc 138:2762-2768. https://doi.org/10.1021/jacs.5b12704. 27. Redmond RW, Gamlin JN. 1999. A compilation of singlet oxygen yieldsfrom biologically relevant molecules. Photochem Photobiol 70:391-475. 28. Hoebeke M, Damoiseau X. 2002. Determination of the singlet oxygenquantum yield of bacteriochlorin a: a comparative study in phosphatebuffer and aqueous dispersion of dimiristoyl-L-alpha-phosphatidylcholineliposomes. Photochem Photobiol Sci 1:283-287. https://doi.org/10.1039/b201081j. 29. Wardman P. 1989. Reduction potentials of one-electron couples in-volving free radicals in aqueous solution. J Phys Chem Ref Data18:1637-1755. https://doi.org/10.1063/1.555843. 30. Dalmazio l, Moura FCC, Araújo MH, Alves TMA, Lago RM, de Lima GF,Duarte HA, Augusti R. 2006. The iodide-catalyzed decomposition ofhydrogen peroxide: mechanistic details of an old reaction as revealed byelectrospray ionization mass spectrometry monitoring. J Braz Chem Soc19:1105-1110. 31. Huang L, St Denis TG, Xuan Y, Huang YY, Tanaka M, Zadlo A, Sarna T,Hamblin MR. 2012. Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: role of ambient oxygen and azide radicals. Free Radic Biol Med 53:2062-2071. https://doi.org/10.1016/j.freeradbiomed.2012.09.006. 32. Mosinger J, Mosinger B. 1995. Photodynamic sensitizers assay: rapid andsensitive iodometric measurement. Experientia 51:106-109. https://doi.org/10.1007/BF01929349. 33. Chmyrov A, Sanden T, Widengren J. 2010. lodide as a fluorescencequencher and promoter—mechanisms and possible implications. J PhysChem B 114:11282-11291. https://doi.org/10.1021/jp103837f. 34. Denofrio MP, Ogilby PR, Thomas AH, Lorente C. 2014. Selective quench-ing of triplet excited states of pteridines. Photochem Photobiol Sci13:1058-1065. https://doi.org/10.1039/c4pp00079j. 35. Lambert C, Sarna T, Truscott TG. 1990. Rose Bengal radicals and theirreactivity. J Chem Soc Faraday Trans 86:3879-3882. https://doi.org/10.1039/ft9908603879. 36. Guillamet CV, Kollef MH. 2016. How to stratify patients at risk for resistant bugs in skin and soft tissue infections? Curr Opin Infect Dis 29:116-123.https://doi.org/10.1097/QCO.0000000000000244. 37. Fothergill JL, Winstanley C, James CE. 2012. Novel therapeutic strategiesto counter Pseudomonas aeruginosa infections. Expert Rev Anti InfectTher 10:219-235. https://doi.org/10.1586/eri.11.168. 38. Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. 2003. Opticalmonitoring and treatment of potentially lethal wound infections in vivo.J Infect Dis 187:1717-1726. https://doi.org/10.1086/375244. 39. Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. 2011. Photody-namic therapy for infections: clinical applications. Lasers Surg Med43:755-767. https://doi.org/10.1002/lsm.21080. 40. Szewczyk G, Zadlo A,Sarna M, Ito S, Wakamatsu K, Sarna T. 2016. Aerobicphotoreactivity of synthetic eumelanins and pheomelanins: generationof singlet oxygen and superoxide anion. Pigment Cell Melanoma Res29:669-678. https://doi.org/10.1111/pcmr.12514. July Volume Issue entimicrobial Agents and Chemotherapyaac.asm.org JulyVolume Issue eac.asm.org Rose bengal (RB) is a halogenated xanthene dye that has been used to mediate antimicrobial photodynamic inactivation for several years. While RB is highly active against Gram-positive bacteria, it is largely inactive in killing Gram-negative bacteria. We have discovered that addition of the nontoxic salt potassium iodide (100 mM) potentiates green light (540-nm)-mediated killing by up to 6 extra logs with the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa, the Gram-positive bacterium methicillin-resistant Staphylococcus aureus, and the fungal yeast Candida albicans. The mechanism is proposed to be singlet oxygen addition to iodide anion to form peroxyiodide, which decomposes into radicals and, finally, forms hydrogen peroxide and molecular iodine. The effects of these different bactericidal species can be teased apart by comparing the levels of killing achieved in three different scenarios: (i) cells, RB, and KI are mixed together and then illuminated with green light; (ii) cells and RB are centrifuged, and then KI is added andthe mixture is illuminated with green light; and (iii) RB and KI are illuminated withgreen light, and then cells are added after illumination with the light. We also showed that KI could potentiate RB photodynamic therapy in a mouse model of skin abrasions infected with bioluminescent P. aeruginosa.
关闭-
1/15

-
2/15
还剩13页未读,是否继续阅读?
继续免费阅读全文产品配置单
北京欧兰科技发展有限公司为您提供《碘化钾增强玫瑰红介导中光动力失活特性检测方案(激光产品)》,该方案主要用于其他中光动力失活特性检测,参考标准《暂无》,《碘化钾增强玫瑰红介导中光动力失活特性检测方案(激光产品)》用到的仪器有Ekspla NT200 红外波段可调谐激光器。
我要纠错
相关方案






 咨询
咨询