-
+关注
私聊
-

第32楼2005/04/12
SUMMARY
Hydride generation atomic absorption
spectrometry (HGAAS) is a sensitive and
selective method for the determination of
As(T) and arsenic(III); however, it is subject
to metal interferences for acid mine waters.
Antimony(III) and Sb(V) interfere with the
HGAAS As(T) determination when the molar
ratios of Sb(III) and Sb(V) exceed 4 and 2,
respectively. Low As(III) recoveries occurred
when the molar ratios of metals to As(III)
were: Cu(II) greater than 120, Fe(III) greater
than 70, Cr(VI) greater than 2, Cd greater
than 800, Sb(III) greater than 3, Sb(V) greater
than 12, or Se(IV) greater than 1. Samples
could not be diluted to an As(III)
concentration (As(III) less than 20 μg/L)
below which these interferences were absent.
Copper(II) and Fe(III) are of primary concern
because water generated from acid mine
drainage potentially contains high
concentrations of Cu(II) and Fe(III).
Separation of Fe(III) and Cu(II) from the
sample while maintaining the existing
As(III)/As(T) ratio can be achieved using
cation exchange.
Some acid mine waters contain high
Fe(III) and Cu(II) and accurate measurements
of As(III) is problematic without removing
the Fe(III) and Cu(II) prior to hydride
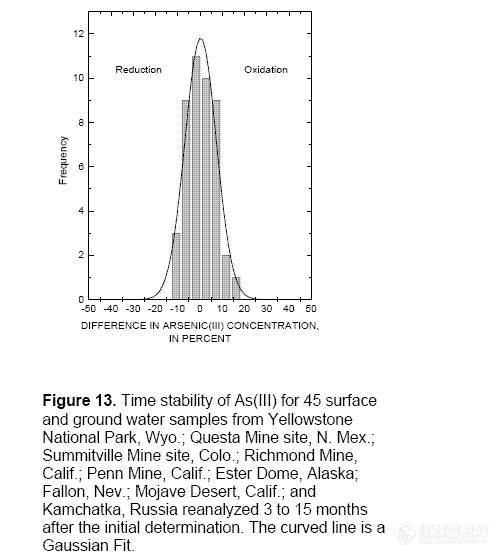
generation. Proper sample collection and
preservation are critical to maintain the
existing As(III/V) ratio prior to the analysis.
Filtering samples through a 0.1 μm filter,
acidifying with HCl to a pH less than 2, and
storing in an opaque bottle at 4°C inhibits
changes in the As(III)/As(T) ratios for up to
15 months after sample collection.
-
+关注
私聊
-

第33楼2005/04/12
REFERENCES CITED
Bio-Rad, 2003, AG 50W and AG MP-50
cation exchange resins, instruction
manual: accessed April 28, 2003 at
http://www.bio-rad.com/webmaster/pdfs/9118_AG_50.pdf
Cherry, J.A., Shaikh, A.U., Tallman, D.E.,
and Nicholson, R.V., 1979, Arsenic
species as an indicator of redox
conditions in groundwater: Journal of
Hydrology, v. 43, p. 373-392.
Creed, J.T., Magnuson, M.L., and Brockhoff,
C.A., 1996, Arsenic determination in
saline waters utilizing a tubular
membrane as a gas-liquid separator for
hydride generation inductively coupled
plasma mass spectrometry: Journal of
Analytical Atomic Spectrometry, v. 11,
p. 505-509.
Emett, M.T. and Khoe, G.H., 2001,
Photochemical oxidation of arsenic by
oxygen and iron in acidic solutions:
Water Resources, v. 35, p. 649-656.
Farrar, J.W., 2000, Results of the U.S.
Geological Survey’s analytical
evaluation program for standard
reference samples distributed in October
1999: U.S. Geological Survey Open-File
Report 00-227, 143 p.
Gao, S., Tanji, K.K., and Goldberg, S., 1988,
Reactivity and transformations of
arsenic: Agroecosystem. Environ.,
[Symp. 75th Annual Meeting Pac. Div.,
Am. Assoc. Adv. Sci.] p. 17-38.
Gihring, T.M., Druschel, G.K., McCleskey,
R.B., Hamers, R.J., and Banfield, J.F.,
2001, Rapid arsenite oxidation by
Thermus aquaticus and Thermus
thermophilus: field and laboratory
investigations: Environmental Science
and Technology, v.35, p. 3857-3862.
Hageman , P.L. and Welsch, E., 1996,
Arsenic, antimony and selenium by flow
injection or continuous flow-hydride
generation-atomic absorption
spectrophotometry: U.S. Geological
Survey Open-File Report 96-525, p. 24-
30.
Hug, S.J., Canonica, L., Wegelin, M.,
Gechter, D. and Von Gunten, U., 2001,
Solar oxidation and removal of arsenic
at circumneutral pH in iron containing
waters: Environmental Science and
Technology, v. 35, p. 2114-2121.
Jamoussi, B., Zafzouf, M., and Hassine, B.B.,
1996, Interferences by transition metals
and their elimination by cyanide as a
complexing agent in the determination
of arsenic using continuous flow HGICP-
AES: Fresenius Journal of Analytical Chemistry, v. 356, p. 331-
334.
-
+关注
私聊
-

第34楼2005/04/12
Nordstrom, D.K. and Alpers C.N., 1999,
Negative pH, efflorescent mineralogy,
and consequences for environmental
restoration at the Iron Mountain
Superfund site, California: Proceedings
National Academy of Science, USA, v.
96, p. 3455-3462.
Smith, A.E., 1975, Interferences in the
determination of elements that form
volatile hydrides with sodium
borohydride using atomic-absorption
spectrophotometry and the argon –
hydrogen flame: Analyst, v. 100, p. 300-
306.
Tan, L.K. and Dutrizac, J.E., 1985,
Determination of arsenic(V) and
arsenic(III) in ferric sulfate-sulfuric acid
leaching media by ion chromatography:
Analytical Chemistry, v. 57, p. 2615-
2620.
To, T.B., Nordstrom, D.K., Cunningham,
K.M., Ball, J.W., and McCleskey, R.B.
1999, New method for the direct
determination of dissolved Fe(III)
concentration in acid mine waters:
Environmental Science and Technology,
v. 33, p. 807-813.
Welsch, E.P., Crock, J.G., and Sanzolone, R.,
1990, Trace-level determination of
arsenic and selenium using continuousflow
hydride generation atomic
absorption spectrophotometry
(HGAAS): U.S. Geological Survey
Open-File Report 90-668, p. 38-45.
Welz, B. and Melcher, M., 1984, Mechanisms
of transition metal interferences in
hydride generation atomic-absorption
spectrometry. Part 2. Influence of the
valence state of arsenic on the degree of
signal depression caused by copper, iron
and nickel: Analyst, v. 109, p. 573-575.
Wilkie, J.A. and Hering, J.G., 1998, Rapid
oxidation of geothermal arsenic(III) in
stream waters of the Easters Sierra
Nevada: Environmental Science and
Technology, v. 32, p. 657-662.
Wilkie, J.A. and Hering, J.G., 1996,
Adsorption of arsenic onto hydrous
ferric oxide; Effect of
adsorbate/adsorbent ratios and cooccurring
solutes: Colloids and Surfaces,
v. 107, p. 97-110.
Wing, R., Nordstrom, D.K., and Parks, G.A.
1987, Treatment of groundwater
samples to prevent loss or oxidation of
inorganic arsenic species: Wing, R.,
1987, Analytical Characterization of
Arsenic in Natural Waters, M.S. Thesis,
Stanford University, p. I-1 – I-25.
- 精
- 该帖子已被管理者-设置为精华,下面是奖励记录:加5积分,加5声望