NEPA21高效基因转染系统
---适用于体外(In Vitro)和活体(In Vivo)

NEPA GENE公司专业研发、生产细胞电转染仪及电融合仪等,其生产CUY21系列电转染仪在研究领域中久负盛名,已被数百篇文献引用,其中不乏高水平杂志的文章,如Nature、Cell、PNAS、Genes & Dvelopment等。
2011年,NEPA GENE公司推出新一代全能型NEPA21高效基因转染系统。与传统的电转仪相比,NEPA21采用全新设计的电转程序,特别适用于难转染细胞、离体组织或动物活体的转染。配合独有的电压衰减(Voltage Decay)设计,NEPA21可在获得高转染效率的同时,提高细胞存活率。
NEPA21高效基因转染系统应用范围十分广泛:
► 对真核细胞进行快速、高效、高存活率的基因转染,特别针对难转染的细胞,如原代细胞、神经细胞、干细胞、悬浮细胞等提供优化的实验方案
► 对贴壁状态的细胞直接进行转染,无需经过细胞消化、悬浮的过程,增加了细胞存活率和实验的简便性
► 对离体的组织或器官进行转染(Ex Vivo Transfection)
► 进行活体动物的基因转染(In Vivo Transfection)
NEPA21高效基因转染系统的优点:
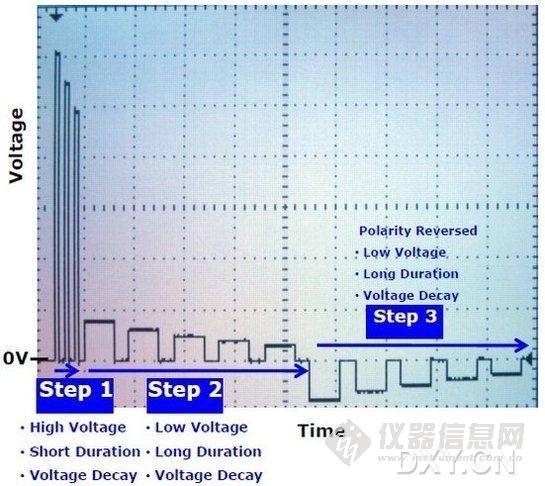
• 全新设计的电转程序
• 特别适用于难转染细胞、离体组织或动物活体的转染
• 适用于DNA和RNA(如siRNA等)转染
• 高转染效率、高细胞存活率
• 电转程序各项参数可见、可调,适用性广
• 不需要特殊的转染试剂盒辅助,运维成本低
应用范围:
♦ 悬浮转染
适用范围:原代细胞、干细胞、以及各种难转染细胞(如免疫细胞、血液细胞等)
♦ 贴壁转染
适用范围:可以直接对贴壁细胞进行转染
♦ 离体组织转染
适用范围:组织切片、脑切片、器官、胚胎等
♦ 活体转染
适用范围:大脑、视网膜、肌肉、皮肤、肝脏、肾脏、睾丸等
♦ 植物细胞转染:
如未去除细胞壁的衣藻的电转:
http://www.instrument.com.cn/netshow/SH102009/news_91877.htm
国内电转DEMO结果(节选):
原代神经元转染效果的新闻报道
http://www.instrument.com.cn/show/news/20120705/079950.shtml
水牛细胞转染效果的新闻报道
http://www.instrument.com.cn/show/news/20120426/077389.shtml
U87 HELA转染效果的新闻报道
http://www.instrument.com.cn/show/news/20120320/075758.shtml
鸡原代输卵管上皮细胞转染效果的新闻报道
http://www.instrument.com.cn/show/news/20120827/081967.shtml
NEPA21作为明星产品亮相各展会:
国际转基因技术协会第十一届世界大会:
http://www.instrument.com.cn/netshow/SH102009/news_92901.htm
2012第五届再生医学和干细胞大会:
http://www.instrument.com.cn/netshow/SH102009/news_86043.htm
厦门-细胞探索完美方案之应用交流会:
http://www.instrument.com.cn/netshow/SH102009/news_86039.htm
国际转基因技术协会第十一届世界大会:
http://www.instrument.com.cn/netshow/SH102009/news_81208.htm
引用文献:
NEPA21
[1].Kobayashi, Y., et al., ERK1/2 mediates unbalanced growth leading to senescence induced by excess thymidine in human cells. Biochemical and Biophysical Research Communications, 2012. 425(4): p. 897-901.
[2].Kusuzawa, S., et al., Leucine-rich glioma inactivated 1 (Lgi1), an epilepsy-related secreted protein, has a nuclear localization signal and localizes to both the cytoplasm and the nucleus of the caudal ganglionic eminence neurons. European Journal of Neuroscience, 2012. 36(3): p. 2284-2292.
[3].Hattori, Y., et al., A selective estrogen receptor modulator inhibits tumor necrosis factor-α-induced apoptosis through the ERK1/2 signaling pathway in human chondrocytes. Biochemical and Biophysical Research Communications, 2012. 421(3): p. 418-424.
[4].Aoyagi, K., et al., Acute Inhibition of PI3K-PDK1-Akt Pathway Potentiates Insulin Secretion through Upregulation of Newcomer Granule Fusions in Pancreatic β-Cells. PLoS ONE, 2012. 7(10): p. e47381
[5].Choijookhuu, N., et al., Estrogen-dependent regulation of sodium/hydrogen exchanger-3 (NHE3) expression via estrogen receptor β in proximal colon of pregnant mice. Histochemistry and Cell Biology, 2012. 137(5): p. 575-587.
[6].Shirakabe, K., et al., VIP36 Protein Is a Target of Ectodomain Shedding and Regulates Phagocytosis in Macrophage Raw 264.7 Cells. Journal of Biological Chemistry, 2011. 286(50): p. 43154 -43163.
CUY21
[1].Katsumura, K.R., S. Maruo and K. Takada, EBV lytic infection enhances transformation of B-lymphocytes infected with EBV in the presence of T-lymphocytes. Journal of Medical Virology, 2012. 84(3): p. 504-510.
[2].Usui, N., et al., Role of motoneuron-derived neurotrophin 3 in survival and axonal projection of sensory neurons during neural circuit formation. Development, 2012. 139(6): p. 1125 -1132.
[3].Yamaguchi, S., et al., Molecular function of microtubule-associated protein 2 for filial imprinting in domestic chicks (Gallus gallus domesticus). Neuroscience Research, 2011. 69(1): p. 32-40.
[4].Iida, A., et al., Dicer Plays Essential Roles for Retinal Development by Regulation of Survival and Differentiation. Investigative Ophthalmology & Visual Science, 2011. 52(6): p. 3008 -3017.
[5].Tachibana, Y., et al., Design and characterization of a polymeric MRI contrast agent based on PVA for in vivo living-cell tracking. Contrast Media & Molecular Imaging, 2010. 5(6): p. 309-317.
[6].Li, Q., et al., Production of human lysozyme-transgenic cloned porcine embryos by somatic nuclear transfer. Progress in Natural Science, 2009. 19(6): p. 699-704.
[7].Furne, C., et al., Netrin-1 is a survival factor during commissural neuron navigation. Proceedings of the National Academy of Sciences, 2008. 105(38): p. 14465 -14470.
[8].Kiyama, S., et al., Reduction of fibrosis in a rat model of non-alcoholic steatohepatitis cirrhosis by human HGF gene transfection using electroporation. Journal of Gastroenterology and Hepatology, 2008. 23(8pt2): p. e471-e476.
[9].ZHANG, K., et al., Effects of Ghrelin on In Vitro Development of Porcine In Vitro Fertilized and Parthenogenetic Embryos. Journal of Reproduction and Development, 2007. 53(3): p. 647-653.
[10].Luo, J., M.J. Ju and C. Redies, Regionalized cadherin-7 expression by radial glia is regulated by Shh and Pax7 during chicken spinal cord development. Neuroscience, 2006. 142(4): p. 1133-1143.
[11].Smith, T.G., et al., Negative feedback predominates over cross-regulation to control ERK MAPK activity in response to FGF signalling in embryos. FEBS Letters, 2006. 580(17): p. 4242-4245.
[12].Blackmore, M. and P.C. Letourneau, L1, β1 integrin, and cadherins mediate axonal regeneration in the embryonic spinal cord. Journal of Neurobiology, 2006. 66(14): p. 1564-1583.
[13].Tada, M., et al., Use of local electroporation enhances methotrexate effects with minimum dose in adjuvant-induced arthritis. Arthritis & Rheumatism, 2005. 52(2): p. 637-641.
[14].Matsunaga, E., et al., RGM and its receptor neogenin regulate neuronal survival. 2004. 6(8): p. 749-755.
[15].Matsuda, T. and C.L. Cepko, Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proceedings of the National Academy of Sciences, 2004. 101(1): p. 16 -22.
[16].Ma, J., et al., Local Actions of Endogenous Angiotensin II in Injured Glomeruli. Journal of the American Society of Nephrology, 2004. 15(5): p. 1268 -1276.
[17].Yoshida, M., et al., Gene Therapy for Central Diabetes Insipidus: Effective Antidiuresis by Muscle-Targeted Gene Transfer. Endocrinology, 2004. 145(1): p. 261 -268.
[18].Yomogida, K., et al., Dramatic Expansion of Germinal Stem Cells by Ectopically Expressed Human Glial Cell Line-Derived Neurotrophic Factor in Mouse Sertoli Cells. Biology of Reproduction, 2003. 69(4): p. 1303 -1307.
[19].Jin, Z., et al., Irx4-mediated regulation of Slit1 expression contributes to the definition of early axonal paths inside the retina. Development, 2003. 130(6): p. 1037 -1048.
[20].Sasagawa, S., et al., Improved mRNA electroporation method for Xenopus neurula embryos. genesis, 2002. 33(2): p. 81-85.
[21].Xue, F., et al., Attenuated acute liver injury in mice by naked hepatocyte growth factor gene transfer into skeletal muscle with electroporation. Gut, 2002. 50(4): p. 558 -562.
[22].Sasagawa, S., et al., Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. genesis, 2002. 33(2): p. 86-96.
更多产品信息,请浏览NEPAGENE英文网址:
世界上首位报道用电转染法进行小鼠胚胎大脑活体转染(Neuroscience.2001;103(4):865-72)的科学家庆应大学Keio University的Dr H Tabata亲自动手演示胚胎活体转染的操作方法,请点击观看视频:
更多详情,请联系各地销售代表
或致电 (50线)
广州市华粤行仪器有限公司为您提供NEPA21 电转仪,nullNEPA21产地为日本,属于电穿孔仪,除了NEPA21 电转仪的参数、价格、型号、原理等信息外,还可为您提供NEPA Porator 双波高效电转系统、NEPA ELEPO21体外高效电转仪、NEPA ECFG21 高效细胞电融合仪 新冠研究,华粤行客服电话,售前、售后均可联系。


 • 全新设计的电转程序
• 全新设计的电转程序



