【飞诺美色谱】EAB10003 Erlotinib Hydrochloride in Plasma
Experimental Details
Sample Preparation
SLE plate procedure
(1) Put the Cleanert SLE plate and 96 well collection plate on the 96-well vacuum manifold
(2) Mix 100 μL of plasma, 10 μL of erlotinib hydrochloride standard aq, 10 μL internal standard solution and 100 μL 10% ammonia together, load the mixture into cartridge
(3) Stand for 5min
(4) Elute with 1400 μL of Methyl-t-butyl ether by gravity, dry with N2, resolve the eluate with 200 μL mobile phase;
LLE procedure
Mix 100 μL of plasma, 10 μL oferlotinib hydrochloride standard aq, 10 μL internal standard solution and 100 μL 10% ammonia together, add the solution into centrifuge tube, then add 1.5mL of Methyl-t-butyl ether into the tube for extraction. The layers were left to separate and the organic aliquot removed, dry with N2, resolve the eluate with 200 μL mobile phase;
Instrumentation
Instrumentation: LC-MS/MS, API 4000
Column: Venusil® Silica, 3×50 mm, 3 μm
Column temperature: 25℃
Mobile phase: 80% ACN aq with 0.1% formic acid, 20% water with 0.1% formic acid
Flow rate: 1 mL/min
Sample Injection: 5 μL
Ion source: ESI – Positive
Scan mode: MRM
| Compounds | Parent ion | Daughter ion |
| Erlotinib hydrochloride | 394.2 | 278.2 |
| Erlotinib hydrochloride -d6 (IS) | 400.2 | 278.2 |
Table 1 MS/MS transitions information of Erlotinib hydrochloride and Erlotinib hydrochloride-d6
Results
(1) Recovery
Same quantity of Methyl-t-butyl is used in the experiment. The follow result shows that the recovery of SLE is higher than that of LLE.
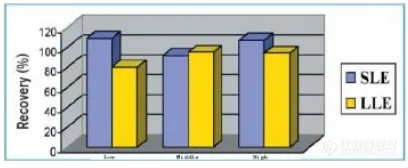
Figure 2 The comparison of recovery between SLE and LLE
(2) Phospholipids Removal
The abundance of phospholipids (496.0/184.0) in plasma sample is detected with LC-MS method. Sample processed with SLE contain 1/100 of phospholipids compared with that processed with LLE.
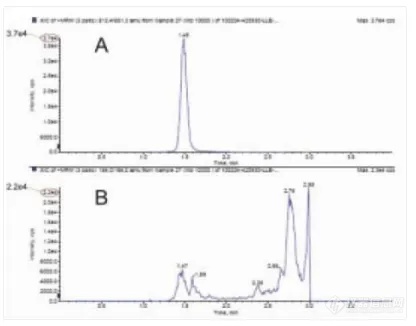
Figure 3 Peak of drug (A) and phospholipids (B) by SLE method
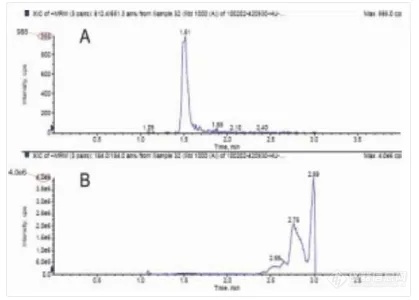
Figure 4 Peak of drug (A) and phospholipids (B) by SPE method
Ordering Information
| Products | Specification | Cat.No |
| Cleanert® SLE plate | 200 mg/well | HC2002Q-9W |
| Venusil® Silica | 3×50 mm, 3 μm | VSi930503-0 |
| 96-well collection plate | 2.2 mL Squaral well | 96SP2036-2 |
| 96-well vacuum manifold | adapt to 96 well plate | VM96 |
| NV-96G for 96 Well Plates | adapt to 96 well plate | NV96-G |
| Acetonitrile | HPLC, 4 L | AH015-4 |
| 1.5 mL vials | 1.5 mL short thread vial, amber glass, labeland filling lines | AV1111-0 |
| 1.5 mL vials caps | 9 mm screw neck cap, center hole; redsilicone/ white PTFE septa | AV2100-0 |
| Micro-insert clear glass | 300 μL micro-insert, 31×6 mm | AV1132-6 |
来源于:艾杰尔飞诺美
热门评论
最新资讯
厂商动态
新闻专题