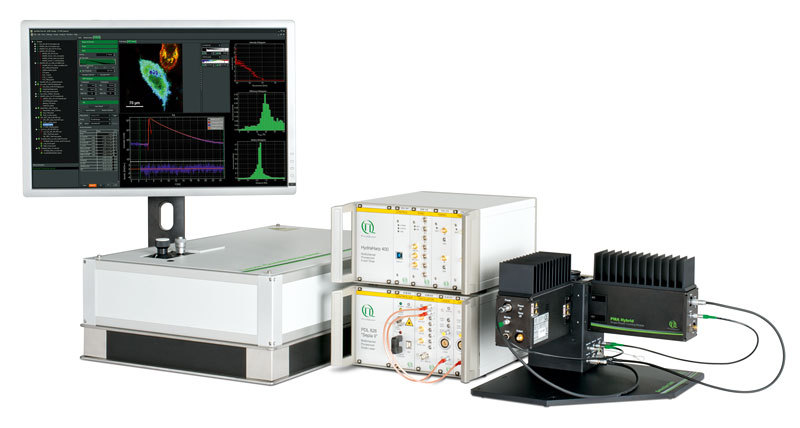
模块化超分辨共聚焦显微系统-LiveCodim传统荧光显微镜受到光学衍射限的影响,高的分辨率为200 nm,因此很难观察细胞中的超微结构。LiveCodim是一款模块化超分辨共聚焦显微系统,能够适配大多数的倒置荧光显微镜,将现有的倒置显微镜升成为具备宽场、共聚焦、超分辨三大模式的成像系统。LiveCodim通过特的锥形衍射显微镜—— 一种强大的波束成形器,能够直接提供分辨率高达120 nm的实时活细胞超分辨共聚焦成像,同时无需对样品进行任何额外操作,结合其低光毒性,以及方便快捷的操作系统等优势,非常适合拍摄荧光成像。产品优势 超高性价比:模块化超分辨,节省成本,兼容大多数倒置显微镜 xy轴超高分辨率:120 nm z轴深度成像:具备z-stack成像能力,高成像深度50 μm 活细胞成像:低光毒性和光漂白性,适合活细胞成像 制样简单:样品无需特殊处理,无需特殊染料 全自动软件:全自动调节各种参数,简单易上手主要参数 xy轴分辨率: 120 nm z轴分辨率: 500 nm z轴成像深度:50 μm 成像视野:共聚焦模式下80 μm * 80 μm,超分辨模式下: 50 μm * 50 μm 成像模式:宽场、共聚焦、LiveCodim超分辨 四色成像通道:405 nm, 488 nm, 561 nm, 640 nm (根据需求可增加)测试数据1. MDCK细胞中线粒体的动态变化2. Hela胞的微管宽场,共聚焦,LiveCodim超分辨成像3. 细胞分裂中期的COS-7细胞3D多色超分辨成像4. 植物细胞成像:观测铃兰草的根茎5. 天然免疫分子TRIM5α作用机制研究天然免疫分子TRIM5α蛋白是人类基因中决定疾病的易感性和发病速度的重要因素,其抗病毒活性通常通过小泛素相关修饰物(SUMO)调节,但是具体的作用机制仍有待进一步研究。LiveCodim超分辨图像揭示了TRIM5α主要分布在肌小管的核膜上,同时与存在于核孔的细胞质丝上的RanGTPase激活蛋白RanGAP1有明显的共定位现象,和主要定位于核篮上的蛋白Nup153无明显共定位,说明TRIM5α主要定位于这类细胞的胞质面。部分发表文章[1] Fernandez, Juliette, et al. "Transportin-1 binds to the HIV-1 capsid via a nuclear localization signal and triggers uncoating." Nature microbiology 4.11 (2019): 1840-1850.[2] Vargas, Jessica Y., et al. "The Wnt/Ca2+ pathway is involved in interneuronal communication mediated by tunneling nanotubes." The EMBO journal 38.23 (2019): e101230.[3] Maarifi, Ghizlane, et al. "RanBP2 regulates the anti-retroviral activity of TRIM5α by SUMOylation at a predicted phosphorylated SUMOylation motif." Communications biology 1.1 (2018): 1-11.[4] Garita-Hernandez, Marcela, et al. "Optogenetic light sensors in human retinal organoids." Frontiers in neuroscience 12 (2018): 789.[5] Getz, Angela M., et al. "Tumor suppressor menin is required for subunit-specific nAChR α5 transcription and nAChR-dependent presynaptic facilitation in cultured mouse hippocampal neurons." Scientific reports 7.1 (2017): 1-16.[6] Portilho, Débora M., Roger Persson, and Nathalie Arhel. "Role of non-motile microtubule-associated proteins in virus trafficking." Biomolecular concepts 7.5-6 (2016): 283-292.[7] Pagliuso, Alessandro, et al. "A role for septin 2 in Drp1‐mediated mitochondrial fission." EMBO reports 17.6 (2016): 858-873.[8] Fallet, Clement, and Gabriel Y. Sirat. "Achromatization of conical diffraction: application to the generation of a polychromatic optical vortex." Optics letters 41.4 (2016): 769-772.[9] Fallet, Clement, et al. "Accurate axial localization by conical diffraction beam shaping generating a dark-helix PSF." Single Molecule Spectroscopy and Superresolution Imaging IX. Vol. 9714. International Society for Optics and Photonics, 2016.[10] Fallet, Clement, Arvid Lindberg, and Gabriel Y. Sirat. "Generating 3D depletion distribution in an achromatic single-channel monolithic system." Single Molecule Spectroscopy and Superresolution Imaging IX. Vol. 9714. International Society for Optics and Photonics, 2016.[11] Fallet, Clément, et al. "A new method to achieve tens of nm axial super-localization based on conical diffraction PSF shaping." Single Molecule Spectroscopy and Superresolution Imaging VIII. Vol. 9331. International Society for Optics and Photonics, 2015.[12] Caron, Julien, et al. "Conical diffraction illumination opens the way for low phototoxicity super-resolution imaging." Cell adhesion & migration 8.5 (2014): 430-439.[13] Fallet, Clément, et al. "Conical diffraction as a versatile building block to implement new imaging modalities for superresolution in fluorescence microscopy." Nanoimaging and Nanospectroscopy II. Vol. 9169. International Society for Optics and Photonics, 2014.[14] Rosset, Sybille, Clement Fallet, and Gabriel Y. Sirat. "Focusing by a high numerical aperture lens of distributions generated by conical diffraction." Optics letters 39.23 (2014): 6569-6572.用户单位 法国巴斯德研究所蒙彼利埃大学
 留言咨询
留言咨询

 400-803-1678
400-803-1678
 留言咨询
留言咨询

 400-628-5299
400-628-5299
 留言咨询
留言咨询

 400-803-1678
400-803-1678
 留言咨询
留言咨询

 400-860-5168转3912
400-860-5168转3912
 留言咨询
留言咨询

 留言咨询
留言咨询

 400-860-5168转0980
400-860-5168转0980
 留言咨询
留言咨询

 400-860-5168转3912
400-860-5168转3912
 留言咨询
留言咨询

 400-860-5168转1980
400-860-5168转1980
 留言咨询
留言咨询
 400-860-5168转3912
400-860-5168转3912
 留言咨询
留言咨询

 400-860-5168转2831
400-860-5168转2831
 留言咨询
留言咨询

 400-807-2969
400-807-2969
 留言咨询
留言咨询

 400-860-5168转6117
400-860-5168转6117
 留言咨询
留言咨询

 400-860-5168转2831
400-860-5168转2831
 留言咨询
留言咨询

 400-860-5168转6117
400-860-5168转6117
 留言咨询
留言咨询

 400-860-5168转2831
400-860-5168转2831
 留言咨询
留言咨询

 400-895-1665
400-895-1665
 留言咨询
留言咨询

 400-860-5168转2826
400-860-5168转2826
 留言咨询
留言咨询

 400-860-5168转6117
400-860-5168转6117
 留言咨询
留言咨询

 400-877-0075
400-877-0075
 留言咨询
留言咨询

 400-860-5168转6117
400-860-5168转6117
 留言咨询
留言咨询

 400-860-5168转4646
400-860-5168转4646
 留言咨询
留言咨询

 留言咨询
留言咨询

 400-860-5168转6097
400-860-5168转6097
 留言咨询
留言咨询

 400-860-5168转2045
400-860-5168转2045
 留言咨询
留言咨询

 400-860-5168转6117
400-860-5168转6117
 留言咨询
留言咨询

 400-860-5168转2045
400-860-5168转2045
 留言咨询
留言咨询

 400-628-5299
400-628-5299
 留言咨询
留言咨询

 400-860-5168转2045
400-860-5168转2045
 留言咨询
留言咨询

 400-860-5168转2831
400-860-5168转2831
 留言咨询
留言咨询

 400-860-5168转3825
400-860-5168转3825
 留言咨询
留言咨询