新华社华盛顿8月8日电 美国研究人员8日在美国《科学转化医学》杂志上报告说,他们已开发出了针对尼帕病毒和亨德拉病毒的高效疫苗。 尼帕病毒及与其关系紧密的亨德拉病毒均为美国国家卫生研究院研究的罕见病毒,它们能攻击人和动物的肺部和大脑,死亡率分别高达75%和60%,而尼帕病毒还可以人际间传播。 研究人员以亨德拉病毒的表面蛋白——G蛋白为基础,研制出新疫苗,这种疫苗可以激发宿主的免疫反应。结果显示,这种疫苗不仅对感染了亨德拉病毒的雪貂和马有效,还能保护感染尼帕病毒的猫。 研究人员进一步对9只非洲绿猴展开了实验,它们被分为3组,每组接种不同剂量的疫苗。42天后研究人员让它们感染了尼帕病毒,结果这些绿猴都得以幸存。 研究论文作者、美国军队卫生服务大学教授克里斯托弗·布罗德表示,这项发现提供了人类感染尼帕病毒或亨德拉病毒的潜在疗法。 研究人员计划下一步搜集更多数据,以便获得美国食品和药物管理局批准,将疫苗应用到人体上。(记者 任海军)
【序号】:【作者】:Joshua T. Y. Tse and H. C. Ong【题名】:Quality factor of plasmonic monopartite and bipartite surface lattice resonances【期刊】:Phys. Rev. B 2021【全文链接】:https://journals.aps.org/prb/abstract/10.1103/PhysRevB.104.125442
【序号】:1【作者】:Xianfeng Yang, Julien Kimmig, Dayou Zhai, Yu Liu, Sara R. Kimmig & Shanchi Peng 【题名】:A juvenile-rich palaeocommunity of the lower Cambrian Chengjiang biota sheds light on palaeo-boom or palaeo-bust environments【期刊】:Nature Ecology & Evolution 【年、卷、期、起止页码】:volume 5, pages1082–1090 (2021)Cite this article【全文链接】:https://www.nature.com/articles/s41559-021-01490-4
ASTM E296-70(Reapproved 1999)Standard Practice for Ionization Gage Application to Space Simulators1[img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=138750]ASTM E296-70(Reapproved 1999)Standard Practice for Ionization Gage Application to Space Simulators1[/url]
[b]Poor comparability of plasma renin activity measurement in determining patient samples: the status quo and recommendations for harmonization[/b]
目的使用ACQUITY UPLC®/Xevo™ G2 QTof质谱系统及MetaboLynx™ XS应用管理软件,鉴定通过人肝微粒体体外孵育而获取的1 μM维拉帕米的代谢物。背景近年来,随着越来越多的一线药品因存在安全性顾虑而退出市场,人们对药品研发过程中的药物代谢和毒性研究给予了更多的关注。如今,在药物发现和研制阶段提早进行药物代谢研究的趋势已比较明显。普遍的做法是对母体药物进行体外代谢物研究,以便在药品开发早期迅速确定其弱点。在药物发现阶段进行代谢物鉴定的一项挑战是:需要提供快速而通用的方法,并且该方法应足够灵敏,以使体外孵育研究可在低μM浓度水平下进行,从而使其更接近于化合物的体内作用情况。一项典型的体外代谢研究还包括分析母体药物的代谢速率和途径。此类研究的理想分析方案需提供在模拟体内条件的底物浓度下对代谢物进行检测的分析速度和灵敏度。利用与UPLC/MSE联用的Xevo G2 QTof质谱系统,体外代谢物研究可在低μM水平下进行,同时具有较好的速度、灵敏度和选择性。http://www.bio-equip.com/imgatl/20115514946.jpg图1. 人肝微粒体维拉帕米(1μM)的孵育结果显示在MetaboLynx浏览器中。解决方案将浓度为1 μM的维拉帕米与人肝微粒体在37°C下进行孵育,并分别在 0、15、30、60、120和 240分钟时加入等体积的冷乙腈终止反应。对样品进行离心,并取上清液直接进样。采用沃特世ACQUITY UPLC®系统,ACQUITY UPLC HSS T3色谱柱(1.7 μm、2.1 x 100 mm),进行色谱分离。流动相由0.1%甲酸水溶液(A)和乙腈(B)组成,进样量为5.0 μL。在ESI正离子模式下,使用Xevo G2 QTof质谱仪采用UPLC/MSE技术进行数据采集,这样一次进样即可同时获取母离子和产物离子的数据。MetaboLynx XS应用管理软件用于进行数据挖掘,结果显示在MetaboLynx浏览器中(如图1所示)。产物离子信息同时进行处理,并显示在MetaboLynx浏览器中的碎片分析窗口内(图2)。通过对多个孵育时间点的样品进样分析,母体药物的清除曲线和代谢物的形成曲线可在同一次试验中同时获取(如图3所示)。http://www.bio-equip.com/imgatl/20115514643.jpg图2. 碎片分析窗口中所显示的MS/MS信息。http://www.bio-equip.com/imgatl/20115514816.jpg图3. 维拉帕米的清除曲线(3A)及其代谢物的形成曲线(3B)。通过采用UPLC/MSE 数据采集策略,再加上具有化学智能的MetaboLynx XS数据处理工作流程,只需进行一次液相色谱进样即可快速完成所有代谢物的鉴定工作。通过在多个时间点进样,可比较容易地获取低浓度(μM)孵育水平下目标药物的代谢速率和途径。因此,产能最大化的目标即可轻松实现。总结这个应用表明:通过使用配备UPLC/MSE 和MetaboLynx XS工作流程的Xevo G2 QTof质谱系统,体外代谢物研究可在低浓度(μM)水平下进行,同时具有较好的速度、灵敏度和选择性。
JIS C 8105-2-12-2009 Luminaires - Part 2-12 Particular requirements- Mains socket-outlet mounted nightlights[img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=174262]JIS C 8105-2-12-2009 Luminaires - Part 2-12 Particular requirements- Mains socket-outlet mounted nightlights.pdf[/url]
怕拉膜就是Parafilm,用的时候要拉一拉。实验室基本上必备。百度:Parafilm® M和分配器Parafilm® M是一种自动封口、可模压、韧性好的特制薄膜产品,广泛用于常规实验室,包括常规电子显微实验室。Parafilm® M具有独特的渗透性能,卓越的水汽通透性能和很强抗腐蚀性能。辅之以特制Parafilm® M分配器,可盛装2" (50.8 mm)或4" (101.6 mm)宽的薄膜卷,用户可方便地切割出同样尺寸的薄膜条或2" (50.8 mm)的薄膜片。所有Parafilm产品的厚度均为0.005" (127µm),尽管有客户询问我们有无其他厚度薄膜,但目前而言尚无其他产品。能轻松打成“死结”是Parafilm的另一全新特点。Parafilm的长度甚至可以拉伸3-4倍而不会发生断裂。Parafilm:Parafilm M实验室密封薄膜系列产品已在全球范围内广泛受到认可。在此基础上,我们又推出了许多新产品以满足部分特殊用户的需求,包括Parafilm芽接(嫁接)带、Parafilm® 花茎裹带和Floratape®等。用不了多久您就能了解不同Parafilm产品之间的差异。 实验室:Parafilm® 是一种热塑性自封薄膜,是科研实验室用理想薄膜。它能够紧紧锁住水分,可对培养基试管、细颈瓶、培养管及陪替氏培养皿中的物质提供完美保护。因该薄膜独特的柔韧性甚至可用于不规则及褶皱表面。即使细颈瓶不小心摔落也不会导致渗溢,方便清洁工作。如果采用Parafilm® M薄膜正确进行密封,容器内液体不会出现任何溢出、污染和蒸发。显微实验室:柔韧性、可模压Parafilm® M为无色、无味、半透明、自动封口热塑薄膜,能轻松覆盖于各种实验室器皿口处。这种薄膜不仅能保持住试管、细颈瓶及陪替氏培养皿内物质的水分,而且可以很好地通透氧气和二氧化碳,因此是实验室必备的理想薄膜产品。在电子显微镜应用中,可用来拾取液体表面的浮置栅极和膜层。 TEM中的独特应用:Parafilm® M可形成憎水表面,让小量试剂附着于TEM网格上。试剂滴落在Parafilm薄膜表面上会形成一颗精致不流散的“水珠”,这时可使网格“接触”试剂提取适量液体。如果试剂昂贵,此法尤为适用。 医院:Parafilm® M在引流过程中可用作高级绷带防护罩,而且在医学实验室及医疗器械存储中有广泛的使用空间。可用作托盘及药品架衬垫以防药瓶、容器及仪器滑落,效果非常理想。Parafilm M可用射线或过氧化氢进行消毒。但我们并不保证Parafilm产品在出厂时已进行消毒处理。园艺:Parafilm薄膜还可独特运用于芽接。它能对创伤处进行密封,保护嫩芽不受雨淋及尘雾摧袭。更重要的是它能帮助锁住水分,防止幼芽干死。注意:请使用最新的Parafilm薄膜产品用于芽接。遮蔽应用:Parafilm可有效应用于各种表面,盖住各种死角,而且非常容易去除,还不会留下任何痕迹。因此,很多人喜欢将其用于遮蔽模具。用于此种用途的Parafilm® M薄膜为背面覆有剥离纸的腊状弹性薄层。推荐使用方法:先从2"或4"薄膜卷上切下一条薄膜,然后慢慢将其拉伸至原长度的四倍左右,保持一分钟(这样能放松薄膜应力,使拉伸后的薄膜产生良好的“粘着力”);此后薄膜便可用于待保护和遮蔽的表面了,通过指压将其压附于基片表面即可产生良好的粘着效果。上漆后,薄膜用牙签轻挑即可剥离,与许多其他遮蔽剂不同,Parafilm绝不会在表面留下任何痕迹。室内植物:该薄膜还可用于各种室内植物。 园艺和普通农业:近年来,Parafilm M 用于密封某些农产品的茎干的需求有所增加,如用于“绿色”香蕉,当它们从植物上割下后,由于使用杀菌剂不符合“绿色”产品的要求,可用该产品保护它们免受霉菌的滋生。 散装Parafilm薄膜:用户有时问我们求购含腊树脂,不是成形产品而是散装产品。由于生产已定型,我们目前只能提供下述四种规格产品。另一问题是要溶解一定量的Parafilm薄膜,哪种溶剂最为合适。对此,我们推荐氯仿。虽然乙基醚也可以,但它本身除了易爆外还有其他危险性。即使用氯仿作溶剂,用户也必须严格进行防范,以防与水汽直接接触。政府核准:据我们所知,Parafilm薄膜是否可用于食品行业还没有经过任何测试,美国食品与药品管理局(FDA)也从未批准过。虽然没有发现Parafilm薄膜成分中有任何有毒物质,但我们仍然需要声明的是其没有用于食品行业的许可。在欧洲,类似声明更为详细。有不少报告证实,该产品会释放出少量的植物毒素,尤其是有丁羟甲苯(BHT)。尽管如此,薄膜生产商告诉我们,Parafilm M薄膜配方中所有材料均已获准可与食物直接接触,甚至有些材料可作为食品添加剂。 如果能够提供更多的产品信息,我们就更有可能开辟新的市场。但问题是我们保护产品不被仿制唯一的途径就是保守Parafilm-M的商业秘密。如果将所有成分公布于众,Parafilm-M产品也就失去了所有保护。因此,关于成分纯度的问题,我们的回答只有Parafilm-M所含成分均已获准可与食物直接接触,甚至有些材料可作为食品添加剂。当然,如果双方有保密协议的话,我们可以给出某些机密信息,但这需视情况而定。 介电性:对于Parafilm® M薄膜的介电性,我们还未取得任何数据。但用户经常问及这一问题,根据该产品成分构成,我们估计其 介电常数应与聚乙烯差不多,即2.2左右。 温度特性:温度超过38°C时, Parafilm薄膜性能开始下降,变得难以展开。性能下降的标志是薄膜粘性提高,以至于不能在无破裂情况下延伸到需要的长度。在超过49°C的温度下,暂时存储一下Parafilm薄膜是可以的,但不推荐这样做。事实上,我们无法引证在何温度值下,薄膜将无法使用。特殊的温度适于特殊的场合,即依靠空间密闭且加热的液体在容器中。如果确实需要较高的温度,我们建议使用DuraSeal实验室密封薄膜。 Parafilm薄膜低温使用范围取决于其所受机械作用力的大小。如果Parafilm薄膜没有任何伸缩、弯曲,其完全可以放入液氮中而不会出现任何问题。像大多数塑料材料一样,我们必须要研究材料在干冰和液氮环境中的脆性。暴露在液氮环境中的Parafilm薄膜,在回复到室温后可恢复其原有性能。 光学性能:Parafilm M在可见区域是半透明的。对于UV曝光,我们能够提供UV透射曲线。 勿接近明火!尽管Parafilm® M薄膜使用特制“腊”为原料,但毕竟是腊质。因此性质易燃,使用时请勿靠近本生灯等明火。保存期:Parafilm® M薄膜虽呈惰性,但任何有机物都不能“永久”保存。在7°C至38°C、50%相对湿度的存储环境中,至少可保存三年不会发生变性。
【序号】:1 【作者】: 【题名】:The volatile composition, aroma profile and antioxidant capacity of Yijiangzi (Aatragalus sinicus L.) monofloral honey and its correlation with the flower 【DOI】:10.1016/J.LWT.2024.116565 【年、卷、期、起止页码】: 【全文链接】:
3. Handling, Calculations, Preparation, and StorageThis section contains basic information on the handling, preparation and storage of standards as well as basic calculations and nomenclature.Handling Observing the following recommendations will save considerable time, money, and frustration:Never put solution transfer devices into the standard solution. This precaution avoids possible contamination from the pipette or transfer device.Always pour an aliquot from the standard solution to a suitable container for the purpose of volumetric pipette solution transfer and do not add the aliquot removed back to the original standard solution container. This precaution is intended to avoid contamination of the stock standard solution.Perform volumetric pipette solution transfer at room temperature. Aqueous standard solutions stored at 'lower' temperature will have a higher density. Weight solution transfers avoid this problem provided the density of the standard solution is known or the concentrations units are in wt./wt. rather than wt./volume.Never use glass pipettes or transfer devices with standard solutions containing HF. Free HF attacks glass but it is sometimes considered safe to use glass when the HF is listed as trace and/or as a complex. However, many fluorinated compounds will attack glass just as readily as free HF.Don't trust volumetric pipette standard solution transfer. Weigh the aliquot of the standard taken. This can be easily calculated provided the density of the standard solution is known. There are too many possible pipetting errors to risk a volumetric transfer without checking the accuracy by weighing the aliquot.Uncap your stock standard solutions for the minimum time possible. This is to avoid transpiration concentration of the analytes as well as possible environmental contamination.Replace your stock standard solutions on a regular basis. Regulatory agencies recommend or require at least annual replacement. Why is this precaution taken in view of the fact that the vast majority of inorganic standard solutions are chemically stable for years? This is due to the changing concentration of the standard through container transpiration and the possibility of an operator error through general usage (more info). A mistake may occur the first time you use the stock standard solution or it may never occur with the probability increasing with use and time. In addition, the transpiration concentration effect occurs whether the standard solution is opened / used or not and increases with use and increased vapor space (transpiration rate is proportional to the ratio of the circumference of the bottle opening to vapor space).Calculations The concentration units for chemical standard solutions used for ICP applications are typically expressed in 礸/mL (micrograms per milliliter) or ng/mL (nanograms per milliliter). For example, a 1000 礸/mL solution of Ca+2 contains 1000 micrograms of Ca+2 per each mL of solution and a 1 礸/mL solution of Ca+2 contains 1000 ng of Ca+2 per milliliter of solution. To convert between metric concentration units the following conversions apply:Table 3.1: Mass portion of concentration unit where g = gramPrefix Scientific Notation Decimal equivalents Example Units kilo- (k) = 103 = 1000 g kilogram (kg) milli- (m) = 10-3 = 0.001 g milligram (mg) micro- (? = 10-6 = 0.000001 g microgram (礸) nano- (n) = 10-9 = 0.000000001 g nanogram (ng) pico- (p) = 10-12 = 0.000000000001 g picogram (pg) Table 3.2: Volume portion of concentration unit where L = literPrefix Scientific Notation Decimal equivalents Example Units milli- (m) = 10-3 = 0.001 L milliliter (mL) micro- (? = 10-6 = 0.000001 L microliter (礚) nano- (n) = 10-9 = 0.000000001 L nanoliter (nL) pico- (p) = 10-12 = 0.000000000001 L picoliter (pL) The difference between ppm and 礸/mL is often confused. A common mistake is to refer to the concentration units in ppm as a short cut (parts per million) when we really mean 礸/mL. One ppm is in reality equal to 1 礸/g. In similar fashion ppb (parts per billion) is often equated with ng/mL. One ppb is in reality equal to 1 ng/g. To convert between ppm or ppb to 礸/mL or ng/mL the density of the solution must be known. The equation for conversion between wt./wt. and wt./vol. units is:(礸/g) (density in g/mL) = 礸/mLand/or(ng/g) (density in g/mL) = ng/mLTherefore, if we have a solution that is 1000 礸/mL Ca+2 and know or measure the density to be 1.033 g/mL then the ppm Ca+2 = (1000 礸/mL) / (1.033 g/mL) = 968 礸/g = 968 ppm.When making dilutions the following equation is useful:(mLA)(CA) = (mLB)(CB)For example, to determine how much of a 1000 礸/mL solution of Ca+2 required to prepare 250 mL of a 0.3 礸/mL solution of Ca+2 we would use the above equation as follows:(mLA)(1000 礸/mL) = (250 mL)(0.3 礸/mL)(mLA) = [(250 mL)(0.3 礸/mL)]/ (1000 礸/mL)(mLA) = 0.075 mL = 75 礚Preparation Weight ≠ Volume: Standard chemical solutions can be prepared to weight or volume. The elimination of glass volumetric flasks may be necessary to eliminate certain contamination issues with the use of borosilicate glass or to avoid chemical attack of the glass. It is often assumed that 100 grams of an aqueous solution is close enough to 100 mL to not make a significant difference since the density of water at room temperature is very close to 1.00 (0.998203 at 20.0 °C). Diluting / preparing standard solutions by weight is much easier. Still, the above assumption should not be made. The problem is that trace metals standards are most commonly prepared in water + acid mixtures where the density of the common mineral acids is significantly greaten than 1.00. For example, a 5% v/v aqueous solution of nitric acid will have a density of ~1.017 g/mL which translates into a fixed error of ~1.7%. Higher nitric acid levels will result in larger fixed errors. This same type of problem is true for solutions of other acids to a degree that is a function of the density and concentration of the acid in the standard solution as described by the following equation (to be used for estimation only):dS = [(100-%) + (dA)(%)] / 100Where:dS = density of final solution% = The v/v % of a given aqueous acid solutiondA = density of the concentrated acid usedFor example, lets estimate the density of a 10% v/v aqueous solution of nitric acid made using 70% concentrated nitric acid with a density of 1.42 g/mL.DS = [(100-%) + (dA)(%)]/100 = [(100-10) + (1.42)(10)]/100 = (90 + 14.2)/100 = 1.042 g/mL
Uniform Packaging and Labeling Regulation HB130-2009
Uniform Packaging and Labeling Regulation h130-2002
BS EN 13758-2-2003 Textiles — Solar UV protective properties — Part 2 Classification and marking of apparel
【序号】:1【作者】:Catherine E. Boddy , Emily G. Mitchell , Andrew Merdith , and Alexander G. Liu & AFFILIATIONS【题名】:Palaeolatitudinal distribution of the Ediacaran macrobiota【期刊】:Publication: Journal of the Geological Society【年、卷、期、起止页码】:Volume 179 https://doi.org/10.1144/jgs2021-030【全文链接】:https://www.lyellcollection.org/doi/10.1144/jgs2021-030万分感谢,感谢诸位朋友帮助!!!!
因课题需要,急需参考Preparative Chromatography TechniquesApplications in Natural Product Isolation 一书电子版。恳请大家帮助,谢谢!详细信息:【作者】Hostettmann, K., Marston, Andrew, Hostettmann, Maryse【图书标题】Preparative Chromatography TechniquesApplications in Natural Product Isolation 2nd【出版社】springer【出版日期】1998年第2版【ISBN】978-3-540-62459-2【链接地址或数据库】http://www.springer.com/east/hom ... lsPage=ppmmedia|toc【求助者联系方式】mywang@sjtu.edu.cn【偿付积分】60分
An official from China's manned space program said Friday they were confident that space lab module Tiangong-1 would function until the end of its design life of two years.Tiangong-1 remains in orbit after its involvement in the country's first-ever space docking mission with another unmanned spacecraft this month."Monitoring data show that all the systems of Tiangong-1 are in perfect condition, so we are quite confident that it will work till next year and the end of its design life," said Wang Zhaoyao, deputy director of China's manned space program office, at a press conference on the docking mission of Tiangong-1 and unmanned spacecraft Shenzhou-8.p Tiangong-1, which blasted off into space on Sept. 29, will remain in orbit to await docking attempts by both Shenzhou-9 and Shenzhou-10 in 2012. At least one of the two space vessels will take astronauts.
因课题需要,急需参考Preparative Chromatography TechniquesApplications in Natural Product Isolation 一书电子版。恳请大家帮助,谢谢!详细信息:【作者】Hostettmann, K., Marston, Andrew, Hostettmann, Maryse【图书标题】Preparative Chromatography TechniquesApplications in Natural Product Isolation 2nd【出版社】springer【出版日期】1998年第2版【ISBN】978-3-540-62459-2【链接地址或数据库】http://www.springer.com/east/hom ... lsPage=ppmmedia|toc【求助者联系方式】mywang@sjtu.edu.cn【偿付积分】60分
因课题需要,急需参考Preparative Chromatography TechniquesApplications in Natural Product Isolation 一书电子版。恳请大家帮助,谢谢!详细信息:【作者】Hostettmann, K., Marston, Andrew, Hostettmann, Maryse【图书标题】Preparative Chromatography TechniquesApplications in Natural Product Isolation 2nd【出版社】springer【出版日期】1998年第2版【ISBN】978-3-540-62459-2【链接地址或数据库】http://www.springer.com/east/hom ... lsPage=ppmmedia|toc【求助者联系方式】mywang@sjtu.edu.cn【偿付积分】60分
单位打算买一台红外-拉曼,目前是尼高力的红外6700+拉曼DXR和布鲁克的红外V70+拉曼Senterra在PK。尼高力的红外市场占用率高,整体上要经济实惠,而且本人以前读书也用过他们的东西,这是他们的优势。布鲁克的设备了解不是很多,只知道XRD很牛,请大家给点建议,谢谢!!!
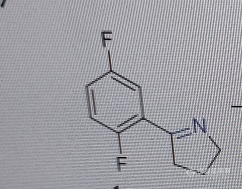
各位大佬 检测过普拉提尼含量吗》? [url=https://insevent.instrument.com.cn/t/Mp]气相色谱[/url]出峰很好 但无法分离底物,,液相也是很难分离底物,[img=,286,227]https://ng1.17img.cn/bbsfiles/images/2021/07/202107241132332101_3417_3150699_3.png!w286x227.jpg[/img][img=,242,189]https://ng1.17img.cn/bbsfiles/images/2021/07/202107241131527937_2159_3150699_3.png!w242x189.jpg[/img]
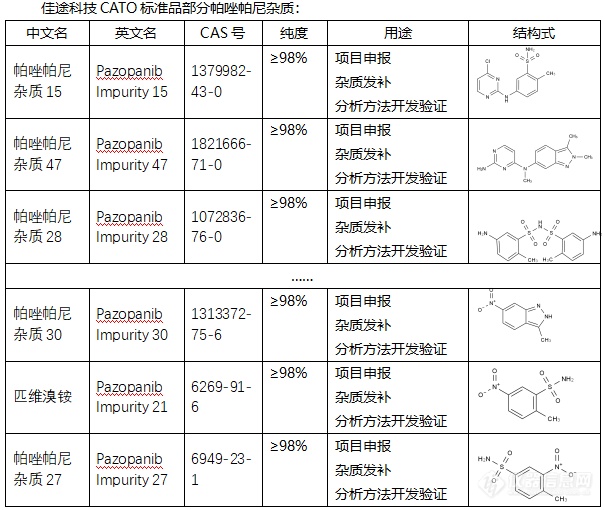
◇帕唑帕尼杂质 帕唑帕尼杂质是在帕唑帕尼药物制备或存储过程中可能产生的物质。帕唑帕尼杂质有多种,其中一些具有特定的CAS号、化学式和分子量。例如,帕唑帕尼杂质(Pazopanib Impurities)的CAS号为59816-94-3,化学式为C22H22N8,分子量为398.46。此外,帕唑帕尼杂质还包括一些异构体和其他结构类似物,如Pazopanib Isomer等。 CATO标准品提供的帕唑帕尼全套的杂质,这些杂质对于药物的纯度和稳定性研究至关重要,也是药物研发过程中不可或缺的一部分。[img=,605,510]https://ng1.17img.cn/bbsfiles/images/2024/02/202402192050040241_6306_6381607_3.png!w605x510.jpg[/img] 广州佳途科技股份有限公司深知药物研发与质量控制的重要性,CATO标准品厂家,提供帕唑帕尼全套的杂质,为客户提供更加精准、可靠的分析标准品,助力药物研发事业的快速发展,以满足客户在药物研发和质量控制方面的需求。
【作者】: 【题名】:Online rapid total nitrogen detection method based on UV spectrum and spatial interval permutation combination population analysis【期刊】:【年、卷、期、起止页码】:【全文链接】:https://www.sciencedirect.com/science/article/abs/pii/S1386142522001573
【序号】:1 【作者】:Aihua Yang a 1, Cui Luo b 1, Jian Han c, Andrey Yu. Zhuravlev d, Joachim Reitner e f, Haijing Sun b, Han Zeng b, Fangchen Zhao b, Shixue Hu g 【题名】:Niche expansion of archaeocyaths during their palaeogeographic migration: Evidence from the Chengjiang Biota 【期刊】:Palaeogeography, Palaeoclimatology, Palaeoecology 【年、卷、期、起止页码】:Volume 653, 1 November 2024, 112419 https://doi.org/10.1016/j.palaeo.2024.112419 【全文链接】:https://www.sciencedirect.com/science/article/abs/pii/S0031018224004085 万分感谢,感谢诸位朋友帮助!!!!
ASTM D5420-21 Standard Test Method for Impact Resistance of Flat, Rigid Plastic Specimen by Means of a Striker Impacted by a Falling Weight (Gardner Impact)
Success in conducting many Pharmacopeial assays and tests depends upon the utmost cleanliness of the glassware apparatus used. For example, the accuracy of the assays of heparin sodium and vitamin B12 activity, as well as the pyrogen and total organic carbon tests, are particularly dependent upon scrupulously clean glassware.One effective method used in the past for cleaning glassware is the application of hot nitric acid. A second traditional method for removing organic matter that does not require heat is the use of a chromic acid–sulfuric acid mixture. However, the chromic acid wash is not recommended because of the hazardous and toxic nature of the material.Several safer alternatives, including the use of cleansing agents, such as trisodium phosphate and synthetic detergents, have proven highly useful, but require prolonged rinsing. It may be useful to rinse with diluted nitric or sulfuric acid prior to rinsing with water. This operation will facilitate removal of residual alkaline material.For optical measurements, special care is required for cleaning containers, but the use of both chromic acid and highly alkaline solutions should be avoided.Effective removal of organic matter is very important for testing pharmaceutical waters in accordance with the general test chapter Total Organic Carbon 643 . It has been demonstrated that an alkaline detergent with potassium hydroxide as the primary ingredient* leaves the least amount of organic matter residuals. Heating in a muffle furnace produces comparable results and is the least labor-intensive procedure however, it requires specialized equipment.In all cases, it is important to verify that the cleaning procedure is appropriate for the particular test or assay being undertaken. This can be accomplished via blank runs, scientific judgments, residuals data from cleansing agent and detergent manufacturers, or other controls. Specifically, special care is required for cleaning containers for optical measurement applications the use of highly alkaline and the no longer recommended chromic acid solutions should be avoided. Finally, a statement should be included in the cleaning protocol describing how the success of the cleaning procedure will be assessed.
ASTM E123-02Standard Specification for Apparatus for Determination of Water by Distillation1[img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=135726]ASTM E123-02Standard Specification for Apparatus for Determination of Water by Distillation1[/url]
ASTM E123-02Standard Specification for Apparatus for Determination of Water by Distillation1[img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=135912]ASTM E123-02Standard Specification for Apparatus for Determination of Water by Distillation1[/url]
[font=&]【题名】:Ionogel-based hybrid polymer-paper handheld platform for nitrite and nitrate determination in water samples[/font][font=&]【全文链接】: [/font]https://www.sciencedirect.com/science/article/pii/S0003267022003245

帕拉科荧光检测器,(流比,闪烁)http://ng1.17img.cn/bbsfiles/images/2013/03/201303221859_431862_1895257_3.jpg
【作者】: 【题名】: Particulate Matter Removal by Filtration and Sedimentation【期刊】:【年、卷、期、起止页码】:【全文链接】:https://onlinelibrary.wiley.com/doi/abs/10.1002/047147844X.mw406