题目Development and Application of a Reversed-Phase High-Performance Liquid Chromatographic Method for Quantitation and Characterization of a Chikungunya Virus-Like Particle Vaccine链接https://pubmed.ncbi.nlm.nih.gov/25234500/
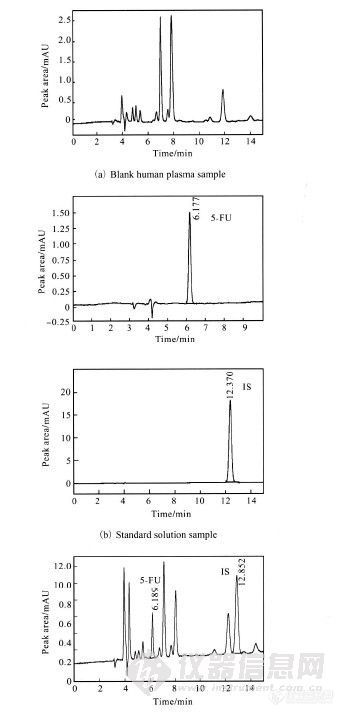
Determination of 5-Fluorouracil in Human Plasma by High-Performance Liquid Chromatography (HPLC) GU Yuan(谷 元)1,2,LU Rong(陆 榕)2 ,SI Duanyun(司端运)2,LIU Changxiao(刘昌孝)2(1. School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China;2. Tianjin State Key Laboratory of Pharmacokinetics and Pharmacodynamics,Tianjin Institute of Pharmaceutical Research, Tianjin 300193, China)Abstract:5-Fluorouracil (5-FU) has a broad spectrum of anti-tumor activity, widely applied to the treatment of cancers.However, it is necessary to determine the plasma concentration of 5-FU in clinical practice due to its narrowtherapeutic index. Therefore, a simple, economic and sensitive high-performance liquid chromatography (HPLC)method was developed and validated for the determination of 5-FU in human plasma. Ethyl acetate was chosen as extractionreagent. Chromatographic separation was performed on a Diamonsil C18 column (250 mm × 4.6 mm i.d., 5μm) with the mobile phase consisting of methanol and 20 mmol/L ammonium formate using a linear gradient elutionat a flow rate of 0.8 mL/min. 5-FU and 5-bromouracil (5-BU) were detected by UV detector at 265 nm. The calibrationcurve was linear over the concentration range of 5—500 ng/mL and the correlation coefficient was not less than0.992 6 for all calibration curves. The intra- and inter-day precisions were less than 10.5% and 4.3%, respectively, and the accuracy was within ±3.7%. The recovery at all concentration levels was 80.1±8.6%. 5-FU was stable under possible conditions of storing and handling. This method is proved applicable to therapeutic drug monitoring and pharmacokinetic studies of 5-FU in human.Keywords:5-fluorouracil(5-FU); high-performance liquid chromatography (HPLC); human plasmahttp://ng1.17img.cn/bbsfiles/images/2012/07/201207232336_379316_2355529_3.jpg
【题名】: Dynamic optical beam shaping system to generate Gaussian and top-hat laser beams of various sizes with circular and square footprint for Additive Manufacturing applications 【链接】: DOI:10.1016/j.procir.2022.08.134
Vip用户 hardman75 在 全站 被 禁止发帖 7 天,被封原因:广告。系统将会在 2010-9-15 自动解封,如有什么意见,请在投诉建议版投诉,特此通告!! --------仪器论坛管理员
求助:书名:Pharmaceutical Computer Systems Validation: Quality Assurance, Risk Management and Regulatory Compliance出版号:ISBN 9781420088946 - CAT# H8894出版时间:February 23, 2010 by CRC Press 其他信息:Professional - 798 Pages - 320 B/W Illustrations作者:Guy Wingate版次:第二版简介:Summary Thoroughly revised to include the latest industry developments, the Second Edition presents a comprehensive overview of computer validation and verification principles and how to put them into practice. To provide the current best practice and guidance on identifying and implementing improvements for computer systems, the text extensively reviews regulations of pharmaceuticals, healthcare products, blood processing, medical devices, clinical systems, and biotechnology. Ensuring that organizations transition smoothly to the new system, this guide explains how to implement the new GMP paradigm while maintaining continuity with current practices. In addition, all 24 case studies from the previous edition have been revised to reflect the new system. Key topics in Pharmaceutical Computer Systems Validation, Second Edition include:GAMP5, ASTM 2500, EU GMP (Annex 11), and US GMP revisions to regulatory requirements for electronic records and signatures that should be published in 2008ICH Guidance Q8, Q9, and Q10 expectationsFDA cGMPs for the 21st Century Initiative and associated guidancePIC/S Guidance on Good Practice for Computerized Systems in GxP EnvironmentsWK9864 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipmentthe indirect developments from FDA/EU/Japan regulators and industrythe role of QA department, and internal and external suppliersthe integration of computer systems validation into single overall approach for wider systempractical guidance on handling common high, medium, and low risk issues that can occur during the life cycle of a computer systemmanaging outsource partners and handling legacy systemstopical issues uncovered by regulatory authorities including US FDA 相关链接:http://www.crcpress.com/product/isbn/9781420088946
![[分享]Good Manufacturing Practices for Pharmaceuticals](https://ng1.17img.cn/bbsfiles/images/2006/08/200608160824_23809_1237095_3.jpg)
ISBN: 0824704258Title: Good Manufacturing Practices for Pharmaceuticals: A Plan for Total Quality Control from Manufacturer to Consumer, 5th edition (Drugs and the Pharmaceutical Sciences: a Series of Textbooks and Monographs)Author: Sidney H. WigPublisher: Informa HealthcarePublication Date: 2000-11-15Number Of Pages: 732Average Amazon Rating: 5.0[img]http://ng1.17img.cn/bbsfiles/images/2006/08/200608160824_23809_1237095_3.jpg[/img]Good Manufacturing Practices for Pharmaceuticals: A Plan for Total Quality Control from Manufacturer to Consumer, 5th editionSummary:Highlighting key issues and differences among GMPs of Europe, Canada, and the WHO, this reference examines US law and governmental policy affecting domestic and multinational pharmaceutical manufacturing. The book recommend pragmatic ways to interpret and comply with FDA CGMP regulation and related criteria. They focus on geographical redistribution of manufacturing facilities, accommodation of a diversity of regulatory and statutory governance, adaptation to disparate human resources, and new growth areas of manufacture and distribution of homeopathic remedies and dietary supplements, in addition to the greater quality control required of pharmacists and other authorized dispensers.This newly revised and expanded reference examines United States law and governmental policy affecting domestic and multinational pharmaceutical manufacturing, recommending pragmatic ways to interpret and comply with FDA Current Good Manufacturing Practice (CGMP) regulation and related criteria, and focusing on geographical redistribution of manufacturing facilities, accommodation of a diversity of regulatory and statutory governance, adaptation to disparate human resources, and new growth areas of manufacture and distribution of homeopathic remedies and dietary supplements, in addition to the greater quality control required of pharmacists and other authorized dispensers. Fifth Edition covers cross-licensing, joint ventures, strategic alliances, mergers, acquisitions, and divestitures that emphasize the necessity of maintaining quality control!Adds descriptions of the Malcolm Baldrige National Quality Award and the ISO 9000 that drive customer satisfaction. [img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=23810]download[/url]
【序号】:1【作者】:Alejandro Izquierdo-López, Jean-Bernard Caron【题名】:BurgessShalemandibulatearthropodwithapygidiumacaseofconvergentevolution【期刊】:Papers in Palaeontology【年、卷、期、起止页码】:First published: 15 June 2021 https://doi.org/10.1002/spp2.1366【全文链接】:https://onlinelibrary.wiley.com/doi/10.1002/spp2.1366万分感谢,感谢诸位朋友帮助!!!!
HPLC is a popular method of analysis because it is easy to learn and use and is not limited by the volatility or stability of the sample compound. The history section illustrates the HPLC's evolution from the 1970's to the 1990's. Modern HPLC has many applications including separation, identification, purification, and quantification of various compounds. It is important for those using HPLC to understand the theory of operation in order to receive the optimum analysis of their compounds. For those interested in purchasing or using an HPLC we have included a list of manufacturers, a troubleshooting guide, technical assistance, and a bibliography to help reduce your personal research and referencing time. Once you have completed the theory of operation, you will be qualified to take a quick quiz to test your understanding of HPLC systems.

LSR4(哈曼法/赛贝克效应/电阻率)http://ng1.17img.cn/bbsfiles/images/2016/01/201601151419_581968_3060548_3.jpg特点、直接测量ZT值+ 可用以计算热传导系+ 高准确度 (使用双样品校正模式)赛贝克系数:静态直流法电阻:四端法ZT:哈曼法用哈曼法测定热电优值是通过样品上(在直流电和绝热条件下)的热电压与欧姆电势降的比值来实现的。在样品中通直流电则相应的“欧姆”压降可直接测得。因为珀尔贴效应,样品一端会被加热而另一端会被冷却,即在样品中产生温度梯度。通过测量产生的压降和热电压,ZT值便可直接得到。LSR—4测试系统可以同时测量塞贝克系数和电阻(电阻率)可以测量圆柱形或棱柱形的样品,长度6——23毫米利用独特的测量适配器可以测量线状和薄片状样品通过三种可更换的炉体,测量温度范围可以覆盖-100到1500 ℃样品架的设计保证了极好的测量重复性最先进的32位软件可以通过程序实现自动测量测量数据导出测量原理:圆柱形或棱柱形的试样垂直放置的两个电极之间,下部电极块包含一个加热器。整个测量装置放置在炉体中。将整个炉体和样品加热到特定的温度,在此温度下利用电极块中的二级加热器建立一组温度梯度,然后两个接触热电偶测量温度梯度T1和T2。独特的热电偶接触机制保证了以最高的温度精度测量每个热电偶上每条导线电动势dE。
Everyone can't bear staying alone for a long time. People should have friends. Do you many friends? There is a saying, "Stranger think I was quiet, my friends think I am very cheerful, my best friend know that I is a lunatic."
我用的HACH启动慢,需要花3-4min大家遇到过没有??是怎么个回事。当购回来时,启动1min就OK啦。现在特别慢,初步怀疑数据太多所以启动慢,但后来看数据根本没有保存,具体原因不详。请有经验的仁兄帮忙指点。
大家有没有用过Whatman NO系列滤纸 ,不同规格滤纸要求怎么样?
[b][color=#f10b00][size=3]经过统计最后得分为:12分【有效回复11个帖子,加11分;经典回复帖子(如下图)1个,多加1分,共计12分】[/size][/color][/b][u][b]备注:[/b][/u][b]1.samanthalas个人发帖情况见:[url]http://www.instrument.com.cn/ilog/bbsarticle.asp?username=samanthalas&PaperType=&FromDate=&strYear=2010&forumid=&page=16[/url]2..经典回复汇总:[img]http://ng1.17img.cn/bbsfiles/images/2010/04/201004131431_211830_1622024_3.jpg[/img][/b]
Stationary phase effects in reversed-phase liquid chromatography http://www.sciencedirect.com/scidirimg/focus_on.gif http://www.sciencedirect.com/scidirimg/focus_off.gif Journal of Chromatography AVolume 656, Issues 1-2, 17 December 1993, Pages 265-287
一个经典的色谱文献,关于峰形选择性和保留行为的。[img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=64425]Shape_selectivity_in_reversed-phase_liquid_chromatograph[/url]
有没有做UPLC ultra performance liquid chromatography,超高效液相色谱仪。大家互相交流一下!
1. 两者是一回事。ramanshift即为拉曼位移或拉曼频移,频率的增加或减小常用波数差表示,拉曼光谱仪得到的谱图横坐标就是波数wavenumber,单位cm-1。2.两者一回事。拉曼频移ramanshift指频率差,但通常用波数wavenumber表示,单位cm-1,可以说某个谱峰拉曼位移是??波数,或??cm-1。3.在Raman谱中,wavenumber有两种理解,一种是相对波数,这时就等于Ramanshift;另一种是绝对波数(这在荧光光谱中用的比较多),这个绝对波数是与激发波长有关,不同的激发波长得到的绝对波数是不一样的,这时Ramanshift等于(10000000/激发波长减去Raman峰的绝对波数)。所以通常在Raman谱中,wavenumber一般可理解为Ramanshift。
请大家帮帮忙,我又一台仪器没说明书和初始密码Hartmann Braun Advance Optima uras 14微量CO2分析仪谁知道初始密码的,或有什么途径可以知道的,请给我留言,麻烦大家了
[img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=33961]Effect of radial gradient of temperature on the performance of large-diameter high-performance liquid chromatography columns[/url]
[img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=33963]Study of radial compression high-performance liquid[/url]
求助啊,alpha-MnO2的拉曼光谱在580cm-1处的峰对应的是deformation modes of Mn-O-Mn chain in the MnO2 octahedral lattice,如果经过酸处理后这个峰右移了,说明什么问题呢?
准备去泰国了,亲们给点意见哈。跟团,两千多点包双人食宿机票费用,六天五晚,深去港回,靠谱吗?
hahamass提供AS 2000 operating manual.pdfhttp://www.instrument.com.cn/bbs/images/upfile/20077414145.rar
There are a lot of electric-books now,even manufacturers just supply E-manual of instrument. Do you like E-manual, E-book or traditional manual or book? What advantage or disadvantages they have?
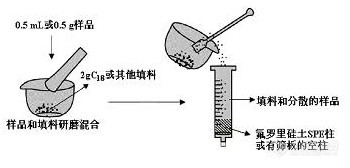
接触固相萃取时间也有点久了,与大家分享一下一点点关于这方面的知识:基体分散固相萃取(MSPD,matrix solid-phase dispersion)是美国Louisiana州立大学的Barker教授在1989年提出并给予理论解释的一种快速样品处理技术。其原理是将涂渍有C18 等多种聚合物的担体固相萃取材料与样品一起研磨,得到半干状态的混合物并将其作为填料装柱,然后用不同的溶剂淋洗柱子,将各种待测物洗脱下来。其优点是浓缩了传统的样品前处理中的样品匀化、组织细胞裂解、提取、净化等过程,不需要进行组织匀浆、沉淀、离心、pH调节和样品转移等操作步骤,避免了样品的损失。MSPD适用于多药物的残留分析,Kandenzki等人以活性弗罗里硅土为填料,利用MSPD技术,测定了26种蔬菜、水果中9类120多种农药残留,回收率大于80%,且与样品的种类无关。它是一种简单高效实际的提取净化方法,适用于各种分子结构和极性农药残留的提取净化,提高了分析速度、减少了试剂用量、适于自动化分析。(摘自百度百科)http://ng1.17img.cn/bbsfiles/images/2012/07/201207081914_376447_1931372_3.jpg有木有童鞋有过这方面的经验呢,也与我们分享一下。。。
In modern world,people can communicate with each other via the internet, can easily reade mail or send email, can upload or download any document, can listen or watch each other via internet. Have you ever had a experience work at home, cummuinate via internet? Or have you had a experience to control your equipments via the internet at home?
【序号】:1【作者】:Johann Harer【题名】:Manufacturing and Quality Assurance in Compliance with the MDR and IVDR【期刊】:Medical Devices and In Vitro Diagnostics【年、卷、期、起止页码】:pp 505–542【全文链接】:https://link.springer.com/referenceworkentry/10.1007/978-3-031-22091-3_20
银量法中佛尔哈德法间接测定Cl- 时, 先加入过量的Ag- ,生成AgCl沉淀, 设法使AgCl凝聚或除去, 以防止影响滴定终点的观察。这句话是正确还是错误,为什么?
请问有哪位大哥知道新 Whatman 阴离子柱的使用与清洗方法?
用佛尔哈德法返滴测Cl-时,为避免因沉淀转化造成的误差,以下所采取的措施错误的是( )。 (A)滴定前将AgCl过滤除去(B)加入淀粉或糊精 (C)加入硝基苯(D)理论终点时轻微摇动