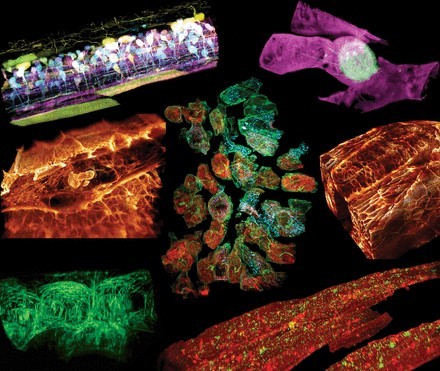
眼见为实|细胞显微学成像技术最新进展盘点
p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " “眼见为实”的行为准则在某种程度上极大促进了人类科学的发展,这也使得科学家们早在1658年就能用显微镜对细胞进行成像。从那时起,显微镜技术已经显著地现代化,并且随着荧光显微镜和三维显微镜的使用,显微镜技术已经成为细胞生物学实验室中无处不在的工具。 /p p style=" text-align: center" img title=" 1.jpg" style=" max-width:100% max-height:100% " alt=" 1.jpg" src=" https://img1.17img.cn/17img/images/201907/uepic/93dd75d7-d021-4ae4-bd52-fc89049c0164.jpg" / /p p style=" margin: 5px 0px text-align: left color: rgb(0, 0, 0) text-transform: none text-indent: 32px letter-spacing: normal font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 text-decoration: none word-spacing: 0px white-space: normal orphans: 2 -webkit-text-stroke-width: 0px background-color: transparent " img title=" " class=" qi_image" style=" margin-bottom: 0px margin-left: 0px margin-right: 0px margin-top: 0px " alt=" " src=" http://qi.mofangyu.com/qi/core/static/ueditor/themes/default/images/spacer.gif" / 看到的信息越多,对细胞的认识便更进一步。随着细胞成像技术的不断发展,人类正朝着能看到更多信息的方向迈进。同时,看到更多的基础上,成像的质量也并不会受损失:显微镜工具可以在更高的分辨率下以惊人的精度获得更多的信息,更重要的是,在最小扰动下活细胞条件下观察。科学家们现在可以常规地可视化单个细胞器,绘制染色体位点的运动图,感知机械力,并连续几天以高通量的方式成像细胞。接下来,将从五个方面介绍细胞显微成像技术的最新进展,以进一步认识该领域的发展方向。 /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " strong 1、布里渊光学显微镜(Brillouin microscopy) /strong /p p style=" text-align: center" img width=" 450" height=" 300" title=" 2.jpg" style=" width: 450px height: 300px max-height: 100% max-width: 100% " alt=" 2.jpg" src=" https://img1.17img.cn/17img/images/201907/uepic/0018117b-acf3-46a3-8384-e1d5e82c2173.jpg" border=" 0" vspace=" 0" / /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 布里渊显微镜是一种非侵入性、无标签的方法,可以在三维空间中以衍射限制分辨率探测生物样品的粘弹性性质。区别于原子力显微镜,它的优点是不接触。在病变组织中,细胞和组织的力学特性经常发生改变,因此对理解病理学机制具有重要意义。布里渊光散射是围绕光与自发热致密度波动的相互作用而展开的。从散射光谱的频移可以推断出诸如刚度的机械特性。这使得许多类型的生物测量成为可能,例如全细胞中细胞内生物力学特性的3D映射,或肠的成像。然而,用这种显微镜技术仍然有待解决的挑战,数据需要特别仔细的解读,因为一些人认为布里渊测量可能主要由水化作用而不是刚度效应来决定的。 /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " strong 2、CRISPR标记荧光成像(CRISPR-labeled fluorescence imaging) /strong /p p style=" text-align: center" img title=" 3.jpg" style=" max-width:100% max-height:100% " alt=" 3.jpg" src=" https://img1.17img.cn/17img/images/201907/uepic/6f3b4fcb-e111-4b48-afca-bd5c987f6424.jpg" / img title=" " class=" qi_image" style=" margin-bottom: 0px margin-left: 0px margin-right: 0px margin-top: 0px " alt=" " src=" http://qi.mofangyu.com/qi/core/static/ueditor/themes/default/images/spacer.gif" / /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " CRISPR无疑已经彻底改变了基因编辑和调控,在这一过程中,它也促进了细胞显微镜技术的发展。与仅对固定细胞成像的常规原位杂交研究相反,研究小组已经用它来标记已定义的染色体位点,以便在活细胞中成像基因组的三维结构。利用Cas9和单导向RNA(sgRNA)结合荧光蛋白,Ma等人最近实现了对单个活细胞中多达6个染色体位点的同步成像,他们称之为CRISPRainbow技术。原则上,他们解释说,只要在CRISPRainbow上再添加一种颜色,就可以将同时检测基因组位点的活细胞数量增加到15个。 /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " strong 3、光片显微镜(Light sheet microscopy) /strong /p p style=" text-align: center" img title=" 4.jpg" style=" max-width:100% max-height:100% " alt=" 4.jpg" src=" https://img1.17img.cn/17img/images/201907/uepic/c01d0f23-99cc-42d6-a10c-5432cdaf96f4.jpg" / br/ /p p style=" margin: 5px 0px text-align: left color: rgb(0, 0, 0) text-transform: none text-indent: 32px letter-spacing: normal font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 text-decoration: none word-spacing: 0px white-space: normal orphans: 2 -webkit-text-stroke-width: 0px background-color: transparent " img title=" " class=" qi_image" style=" margin-bottom: 0px margin-left: 0px margin-right: 0px margin-top: 0px " alt=" " src=" http://qi.mofangyu.com/qi/core/static/ueditor/themes/default/images/spacer.gif" / 光片荧光显微镜(LSFM)只照亮样品的薄成像焦平面,并检测来自该特定平面的荧光,从而最大限度地减少离焦荧光和光漂白。这意味着无需切片就能观察到生物体和细胞的动态。直到最近,分辨率还不允许亚细胞成像的视野大到足以容纳几个细胞。事实上,组织的光学异质性会导致像差,随着成像深度的增加,像差会迅速影响分辨率、信号和对比度。虽然贝塞尔光束和点阵光片已经取得了进展,但这种技术仍然复杂且昂贵。在2019年,Chang等人描述了一种新的场合成方法,它有助于使用更简单的光学器件的光片。这种方法将LSFM与自适应光学相结合,通过改变镜子的形状来产生相等但相反的畸变,从而补偿光学畸变。它需要更少的功率,最大限度地减少光漂白,并允许在同一时间以高分辨率成像多种颜色。这使得Liu等人能够通过检测角蛋白包被的凹坑在人类干细胞衍生的类器官或斑马鱼的背尾区域中的扩散,在纳米尺度上对内吞作用进行成像。并帮助他们在斑马鱼胚胎发生过程中用细节细胞器动态显示,和体内神经元,癌症或免疫细胞的3D细胞迁移。这一突破有望彻底改变定量亚细胞4D细胞生物学。 /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " strong 4、全息断层扫描显微镜(Holo-tomographic microscopy) /strong /p p style=" text-align: center" img title=" 5.jpg" style=" max-width:100% max-height:100% " alt=" 5.jpg" src=" https://img1.17img.cn/17img/images/201907/uepic/584ce9f3-3844-4b8e-a73a-a4ec6c540031.jpg" / /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 全息断层扫描显微镜(HTM)是一种定量相位显微镜方法,其中物体的复杂波场被编码成全息图,并且与样本的旋转扫描相结合。这导致快速三维重建的实时样品的折射率分布的分辨率低于Rayleigh准则定义的光的衍射极限。该技术的一个关键优点是它传输给样品的能量低,确保低光毒性,允许在不受干扰的情况下研究亚细胞动力学。无散射系统现在已经被开发出来,允许亚细胞高分辨率成像,例如通过融合和裂变循环的单个线粒体成像。使用这种技术,Sandoz等人首次报道了小鼠胚胎干细胞(mESCs)有丝分裂前细胞重组的细胞器旋转。在有丝分裂前80分钟,他们观察细胞核、核仁、核膜、脂滴和线粒体的旋转,这表明细胞分裂前物质重新分布的潜在机制有待进一步研究。 /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " strong 5、高内涵分析显微镜(High content-analysis microscopy) /strong /p p style=" text-align: center" img width=" 450" height=" 260" title=" 6.jpg" style=" width: 450px height: 260px max-height: 100% max-width: 100% " alt=" 6.jpg" src=" https://img1.17img.cn/17img/images/201907/uepic/550f8797-8cda-4401-8d5d-24a4e0078abf.jpg" border=" 0" vspace=" 0" / br/ /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 看得更多不仅仅是为了达到高分辨率,它还意味着看得更久。科学家们意识到,短时间成像细胞,或在离散时间点成像细胞,可能意味着它们错过了关键的细胞动力学。这就是为什么现在开发的系统可以对样品进行连续成像。一方面,一些显微镜正在开发中,它们可以集成在孵卵器中,随着细胞不断生长,它们可以在二维时间内追踪细胞。类似地,一些培养箱被设计成包含摄像机,可以对任何测试进行连续成像,从而能够在同一个培养箱中对多个样本进行成像。另一方面,为了设计数据丰富的实验,目前正在开发高通量系统,以便能够长时间地研究孔板中的更多细胞分析。例如,Anastasov等人培养了大量由癌症和基质细胞组成的肿瘤球体,并对其成像14天,以量化它们在不同放疗和化疗组合下的生长。这种高含量的装置使他们能够筛选大量的化疗药物,以及它们与辐射的结合,从而确定长春碱是一种射电致敏剂,与单独使用长春碱的试验相比,它在减小球体大小方面更有效。 /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " strong 相关仪器专场: a style=" color: rgb(0, 176, 240) text-decoration: underline " href=" https://www.instrument.com.cn/list/sort/5.shtml" target=" _blank" span style=" color: rgb(0, 176, 240) " 【光学显微镜专场】 /span /a /strong /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " strong 相关文献 /strong /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 1. Hajdu, S. I. (2003). A note from history: The discovery of blood cells. Ann. Clin. Lab. Sci. 33, 237–8. /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 2. Prevedel R et al. (2019). Brillouin microscopy – a revolutionary tool for mechanobiology? arXiv: 1901.02006. /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 3. Antonacci, G., de Turris, V., Rosa, A. & amp Ruocco, G. (2018). Background-deflection Brillouin microscopy reveals altered biomechanics of intracellular stress granules by ALS protein FUS. Commun. Biol. 1, 139. /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 4. Wu et al. (2018). Water content, not stiffness, dominates Brillouin spectroscopy measurements in hydrated materials. Nat Methods. 15(8):561-562. /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 5. Chen, B. et al. (2013). Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491. /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 6. Ma et al. (2016). Multiplexed labeling of genomic loci with dCas9 and engineered sgRNAs using CRISPRainbow. Nature Biotechnology. 34(5):528-30. /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 7. Forero-Shelton M. (2019). Peering into cells at high resolution just got easier Nat Methods. Apr 16(4). /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 8. Chang, BJ. et al. (2019). Universal light-sheet generation with field synthesis. Nat. Methods 16, 235–238. /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 9. Liu et al. (2018). Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science. 360, 284. /p p style=" background-color: transparent color: rgb(0, 0, 0) font-family: sans-serif font-size: 16px font-style: normal font-variant: normal font-weight: 400 letter-spacing: normal margin-bottom: 5px margin-left: 0px margin-right: 0px margin-top: 5px orphans: 2 text-align: left text-decoration: none text-indent: 32px text-transform: none -webkit-text-stroke-width: 0px white-space: normal word-spacing: 0px " 10. Sandoz P.A et al. (2018). Label free 3D analysis of organelles in living cells by refractive index shows pre-mitotic organelle spinning in mammalian stem cells. BioArxiv. /p p style=" margin: 5px 0px text-align: left color: rgb(0, 0, 0) text-transform: none text-indent: 32px letter-spacing: normal font-family: sans-serif font-size: 16px font-style: normal font-variant-ligatures: normal font-variant-caps: normal font-weight: 400 text-decoration: none word-spacing: 0px white-space: normal orphans: 2 -webkit-text-stroke-width: 0px background-color: transparent " 11. Anastasov, N. et al. (2015). A 3D-microtissue-based phenotypic screening of radiation resistant tumor cells with synchronized chemotherapeutic treatment. BMC Cancer 15, 466. br/ /p