Dy( III ) and Tb( III )中structure, fluorescence, and magnetism?检测方案
检测样品 Dy( III ) and Tb( III )
检测项目 structure, fluorescence, and magnetism?

 白金会员
9 篇解决方案
白金会员
9 篇解决方案
方案详情文
智能文字提取功能测试中
DaltonTransactionsView Article OnlineView Journal|View Issue View Article OnlineDalton TransactionsPaper Chiral six-coordinate Dy(m) and Tb(m) complexesof an achiral ligand: structure, fluorescence, andmagnetismt Mei-Jiao Liu,a Juan Yuan,Yi-Quan Zhang,ID)* Hao-Ling Sun, Cai-Ming Liu dand Hui-Zhong Kou'iD*a Two new chiral six-coordinate lanthanide complexes (1 for Dy and 2 for Tb) have been synthesized usingthe achiral flexible ligand H2L (2,2'-[1,2-ethanediylbis[(methylimino)methylene]]bis[4,5-dimethylphenol]).Both complexes crystallize in the chiral P1 group space, and the enantiomers [Zn(L)CI]3Dy·MeOH0.5H2O(4-1), [Zn(L)CI]3Dy·1.5H2O (A-1), [Zn(L)CI]3Tb1.5H2O (4-2) and [Zn(L)CI]3Tb·H2O (A-2) are isolated in thereaction. Three [Zn(L)CI]-anions coordinate to the central lanthanide ion using two phenoxo oxygenatoms of L-, and the lanthanide ion has the coordination geometry of D3. Complex 1 exhibits a field-induced slow magnetization relaxation, which is typical of a single-ion magnet (SIM). Ab initio calculationson complex 1 and studies on magneto-structural correlationship of six-coordinate Dy(m) SIMs indicatethat the small energy barrier of complex 1 might be related to the absence of a unique anisotropic axis forthe regular octahedral coordination configuration of Dy(iml) and the high quantum tunneling gap betweenthe ground states (mj=+15/2). Complex 2 displays green photofluorescence and triboluminescence. Introduction Since lanthanide ions are used in synthesizing single ionmagnets (SIMs) or single molecule magnets (SMMs), someSIMsandSMMslswwithrhigh-energy bbaarrriershaverebbeenreported.1-The lanthanide ions exhibit multifarious coordi-nation numbers of three to ten, and most of them have beenfound to display SIM or SMM properties.8-22 Recently, theexperimental and theoretical studies suggested that the lantha-nide complexes with weak equatorial and strong axial co-ordinated donors are apt to exhibit good dynamic magneticproperties,23-25aand one reported dysprosium(m) complexshowed the highest energy barrier of 1837 K.26 According tothe literature, most of the major breakthroughs in energy ( “Department ofChemistry, T s inghua University, Beijing 1 00084, P. R. China. ) ( E-mail: kouhz@mail.tsinghua.edu.cn; Tel: +86 1 0 6 2 77 1748 ) ( "Jiangsu Key Laboratory for NSLSCS, School of Physical Science and Technology,Nanjing Normal Un i versity, N a njing 210023, P. R. Ch i na. ) ( E-mail: zhangyiquan@njnu.edu.cnDepartment of Chemistry and Beijing Key Laboratoryof Energy Conversion a ndStorage Materials, Beying Normal University, Beijing Normal University, Beijing 100875, P . R. China “Beijing National Laboratory for Molecular Sciences, C enter for Molecular Science, ) ( Institute of Chemistry, Chinese Academy of Sciences, Be i jing 100190, P. R . ChinatElectronic supplementary in formation ( E SI) available: Additional f igures, crystal date and magnetic data. CCDC 1559740-1 5 59743. For ESI and crystallo-graphic data in CIF or other electronic format see DOI: 10.1039/c7dt02409f ) barrier of SMMs are based on seven-coordinate Dy(m) com-plexes. Low-coordinate lanthanide SMMs ineluding six-coordi-nate lanthanide complexes are still rare.27-29 Although theenergy barrier of a seven-coordinate Dy(m) SIM reported byTong et al. decreases considerably when the coordination geo-metry changes to octahedral,3 the Dys SMM is one goodexample that has a high energy barrier where all Dy(m)ions are six-coordinate.1 The presence of one short Dy-Obond in Dys SMM ensures a favorable electron distributionalong the axial direction, which is responsible for goodmagnetic performance. Given the above findings, we considerthat the study of six-coordinate lanthanide complexes ismeaningful. Bifunctional ormultifunctionalmaterials possessingoptics, electrical property and magnetism have received exten-sive attention for their potential applications in chemistry andbiochemistry.32-36 Bifunctional composites that exhibit bothmagnetism and chirality, can act as chiral molecular magnetsand have received the most attention.32 It is known that chiralcomplexes can be obtained using a chiral ligand via chiralitytransmitting or an achiral1lliigand via spontaneousresolution.37-43 Most reported chiral complexes44-49 were syn-thesized by the former strategy; however, it is confronted withthe problem that the chiral ligand resource is limited. Usingachiral ligands to construct chiral molecular magnets is inter-esting44,45 but challenging due to the possibility of crystalliza-tion of both chiral components forming a racemate. Achiral Scheme 1 Structure of the ligand H2L. bidentate chelating aza-ligands AA are inclined to form propel-ler-like chiral species [M(AA)s]"* of D3 symmetry,5 which arefrequently racemic though.11,51,52 Spontaneous resolution ofchiral[M(AA)3]"+transitinmetal complexeshasbbeenoccasionally achieved.53,54 As far as we are aware, no chiral six-coordinate Ln(m) SMMs have been previously reported. It isworthwhile to investigate spontaneous resolution, magnetismand fluorescence of six-coordinate [Ln(AA)3]" SMMs. To form six-coordinate [Dy(AA)s]"*-type species, the rationalselection of ligands AA is crucial. Recently, Long et al. reportedtwo six-coordinate Dy(BeMe)3 and Dy(BpMe)3 achiral complexes([BeMe]-= dihydrobis(methylimidazolyl)borate, [BpMe]-=dihydro-bis(methypyrazolyl)borate).1 The formation of six-coordinateDy(m) should be due to the steric hindrance of the methylgroups of[BeMe]- and [BpMe]-. Considering the facile tunabi-lity of the N202 flexible chelate ligand H2L (Scheme 1), wechoose [Zn(L)] as the AA ligand to react with lanthanide saltsto explore the possibility of forming six-coordinate lanthanidecomplexes of the [Zn(L)]sLn type. Indeed,two isomers ofZn(II)-Ln(m) (Ln= Dy or Tb) complexes are obtained, andinterestingly spontaneous resolution of the complexes occurs.In this study, we report the synthesis, crystal structure,magnetic and fluorescence properties of two new complexes[Zn(L)CI]sDy·MeOH·0.5H2O (4-1), [Zn(L)Cl]3Dy·1.5HzO (4-1),Zn(L)Cl]3Tb·1.5H2O(4-2) and Zn(L)Cl]sTbH2O(A-2). Experimental Materials All reagents were commercially available and used withoutfurther purification. The ligand HL was synthesized by a pre-viously reported literature method. Synthesis of complexes 1 and 2 Complexes 1 and 2 were synthesized similarly. The ligand HL(0.3 mmol, 107 mg) was suspended in methanol (20 mL), andsolid anhydrous ZnClz (0.3 mmol, 41 mg) was added with stir-ring at room temperature. After the mixture became clear,solid LnClg·6H2O (0.1 mmol) was added into the above solu-tion. Stirring continued for another 15 minutes, and triethyl-amine (0.6 mmol, 84 pL) was slowly injected into the solution.The resultant solution was filtered, and slow diffusion ofdiethyl ether into the filtrate in a test tube afforded colorlessplatelet single crystals for complexes 1 and 2. Yield: about33%. Physical measurements The IR spectra were performed on a WQF-510A FTIR equip-ment. Theffluorescenceesspectrawererecordedon anF98Fluorospectrophotometer.The UV-vis absorption spectra weremeasured withaa TU-1901 spectrophotometer. The powderX-ray diffraction (PXRD) measurements were recorded on aBruker D8 ADVANCE X-ray diffractometer. The solution circu-lar dichroism (CD) spectra were measured with Chirascan plusCD Chiroptical Spectrometer. Temperature and field-depen-dent magnetic susceptibility measurements were carried outon a Quantum Design MPMS-XL5 SQUID or Quantum DesignMPMS-3 SQUID magnetometer. The experimental suscepti-bilities were corrected for diamagnetism of Pascal’s constants.Single-crystal X-ray data were collected on a Rigaku SuperNova,Dual, Cu at zero, AtlasS2. The structure was solved by directmethods (SHELXS-2013) and refined by a full matrix least-squares method based on Fusing SHELXL-2013 or Olex 2 1.2program. Hydrogen atomswere added geometrically andrefined using a riding model. AAb initio computations Complete-active-space self-consistent field (CASSCF) calcu-lations on complex 1 on the basis of X-ray determined geome-try were carried out with MOLCAS 8.2 program package. ForCASSCF calculations, the basis sets for all atoms are atomicnatural orbitalsfromthe MOLCAS ANO-RCC library:ANO-RCC-VTZP for Dy ion; VTZ for close O; VDZ for distantatoms. The calculations employed the second order Douglas-Kroll-Hess Hamiltonian, where scalar relativistic contractionswere taken into account in the basis set and the spin-orbitcoupling was handled separately in the restricted active spacestate interaction (RASSI-SO) procedure. The active electrons in7 active spaces include all felectrons (CAS (9 in 7) for complex1) in the CASSCF calculations. To exclude all the doubts, wecalculated all the roots in the active space. We mixed themaximum number of spin-free state, which was possible withour hardware (all from 21 sextets, 128 from 224 quadrupletsand 130 from 490 doublets). Results and discussion Synthesis Two chiral [Zn(L)Cl]3Ln complexes have been prepared by theone-pot reaction of achiral flexible ligand H2L, ZnCl2, LnClgand Et N in the molar ratio of 3:3:1:6. The colorless crystalsare slightly soluble in MeOH and soluble in DMF. We selecteda few of the single crystals randomly for X-ray diffraction ana-lysis and found that these crystals showed different opticalrotation. For complex 1, among the five single crystals ran-domly chosen from one test tube, three are A-1 and two are4-1 (FLACK parameters are close to nought). Unfortunately, wecould not separate them because the shape and color of thecrystals were very similar and indistinguishable (Fig. S1 andS2, ESIt). When we collected the conglomerate crystals of 4-1and two A-1 for the measurements of the circular dichroism (CD) spectra in methanol, we observed weak CD signals(Fig. S6, ESIt). This phenomenon demonstrates that one of thetwo isomers in the mixture was a major product. Powder XRDpatterns of complexes 1 and 2 are consistent with that calcu-lated from the single crystal data (Fig. S7, ESIt). The UV-visabsorption spectra of complexes 1, 2 and the ZnCl-H2Lmixture in MeOH show that the bands are similar and areslightly red-shifted (bathochromic) in comparison with that offree H2L (Fig. S8, ESIt). The new peaks at 241 nm might bedue to the LMCT of the [Zn(L)Cl] moieties. Crystal structure Complexes 1 and 2 are isostructural and crystallize in the tri-clinic P1 group space. The crystal data and structure refine-ments of complexes 1 and 2 are listed in Table 1. The Ln-Obond distances and O-Ln-O bond angles are listed in Table 2. Complexes 1 and 2 are isostructural and we select A-1 asone example to describe the crystal structure in detail.Complex 4-1 crystallizes in a chiral and polar space group ofP1. As shown in Fig. 1a,A-1 contains two independent [Zn3Dy]molecules, which have the same optical rotation of A, requiredfor the formation of chiral crystals. Each [Zn3Dy] molecule iscomposed of three peripheral [Zn(L)Cl]-fragments and onecentral Dy(m) ion. The Zn(u) ion is penta-coordinated by N202from the flexible ligand L-occupying the basal plane and thechloride anion situated at the apical position. The Zn-Cl bonddistances are 2.209(3)-2.250(3) A for [Zn3Dy(1)] and 2.225(2)-2.249(3) A for [Zn3Dy(2)]. Each Dy(m) is surrounded by three[Zn(L)Cl] anions and coordinated by six phenoxo oxygenatoms of the ligands L-. The bonds distance of Dy(1)-0 andDy(2)-O are in the range of 2.250(5)-2.303(6) A and 2.242(5)-2.311(5) A, respectively. The Dy(m) ion shows a slightly dis-torted octahedral coordination configuration calculated by theSHAPE software with the deviation values of 3.791 for Dy(1) and 3.806 for Dy(2). On the whole, Dy(m) ion has a local sym- Table 22Selected bond distances (A) and bond angles (°) for complexes1 and 2 Complexes 4-1 A-1 A-2 A-2 2.270(6) 2.271(5) 2.294(7) 2.281(8) 2.272(6) 2.278((5) 2.291((7) 2.282(7 2.253(6) 2.248(5) 2.273(6) 2.250((7 2.300(6) 2.291(6) 2.318(6) 2.310(7) 2.259(6) 2.250(5) 2.271(7) 2.264(8) 2.298(6) 2.303((66) 2.333((7) 2.316((7) 2.270(6) 2.277('(55) 2.307((7) 2.295(88) Ln2-O8 2.256(6) 2.284((55) 2.280((7) 2.264(8) 2.300(6 2.311(55) 2.317(7) 2.336( 2.250(5 2.255((55) 2.257(6 2.266(7) 2.299(0(66) 2.291((6 2.321(7) 2.309(8 2.253((66) 2.242((5 2.265(7) 2.253(7) 154.5|(22)- 153.8((22)- 153.8( 153.6(3)- 163.9()(22) 154.0()(22)- 164.4(2) 152.97(19)- 164.4(3) 153.1(3)- 165.11(33) 153.7(3)- 165.3(22) 165.63(19) 165.0(22) 164.0(33) O-Ln1-Ocis 69.1(2)- 69.2(2)- 68.9(2)- 68.8(3)- 104.0(2) 105.8(2) 106.2(3) 107.2(3) 68.8(2)- 68.5(2)- 69.0(3)- 68.6(3)- 104.6(2) 105.3(2) 105.8(3) 104.7(3) metry of D3 similar to that of the M(AA)3-type complexes, e.g.[Co(ox)s]3-.58 The O-Dy-O angles of the six blades are in therange 68.5(2)-72.1(2)°. As shown in Fig. 1c, the two simplifiedenantiomers of complex 1 behave like a three bladed-shapedpropeller and the Dy-Oz-Zn rings are the blades. The shortestdistance between Dyions of adjacent molecules is 13.731 A.The molecular structure of 2co8mplex 1 is similar to that of[NigDy{(py)2C(H)O}](ClO4)3The [NisDy{(py)2C(H)O}]3+cation also has a propeller shape with Ds symmetry, but isracemic in the crystal. Magnetic properties The variable-temperature magnetic susceptibilities of com-plexes 1 and 2 were measured under an external magnetic field Table 1 Crystal data for complexes 1 and 2 Complexes A-1 A-1 4-2 A-2 Formula C67H95Cl3DyN,07.5Zng C66H93Cl3DyN,07.5Zn3 C66H93ClgN,O7.5TbZn, C66H92C13N,OTbZn, Formula weight 1569.44 1555.42 1551.84 1542.83 T(K) 100 173 298 100 Crystal system Triclinic Triclinic Triclinic Triclinic space group P1 P1 P1 P1 a((A 12.4023(4) 12.4388(3) 12.4384(4) 12.3376(3) b(A) 16.1328(5) 16.1852(4) 16.3219(5) 16.2163((4 CA) 19.4673(6) 19.6324(5) 19.7909(6) 19.4135(5) 74.601(3) 74.390(2) 74.076(3) 74.718(2) 82.797([2) 82.638(2) 82.733(2) 82.879(2) Y(9 81.024(2) 80.894(2) 80.874(2) 81.239(2) V((A) 3694.8(2) 3743.46(17) 3800.2(2) 3688.67(17) 2 2 2 2 pcale (g cm3) 1.411 1.380 1.356 1.389 p(mm-1) 2.122 2.094 6.926 2.070 F(000) 1612 1596 1594 1584 Data/restraints/parameters 23902/225/1621 25259/157/1602 26732/760/1621 34214/296/1594 GOF on F 1.036 1.012 1.040 1.042 RiI>2o(I)] 0.0493 0.0430 0.0619 0.0623 wR2 (all data) 0.1305 0.1100 0.1727 0.1507 Fig.1(a) Crystal structure of A-1.(b) Atomic labelling of the core struc-tures of two independent molecules for 4-1. (c) Simplified structures ofA-1 and A-1 isomers. of 1000 Oe. As shown in Fig. 2, the xmT values of 1 and 2 atroom temperature are 14.8 cm³K mol-1 and 10.8 cm’K mol-1,respectively, comparable to the theoretical values of 14.2 and11.8 cm K mol-1 for Dy(m) (H15/2) and Tb(m) (F), respect-ively. The xmT values decrease slowly upon cooling until 50 Kis reached and then they decrease rapidly. At 2 K, the XmTreaches the minimum value of 4.7 cm’K mol- for complex 1and 2.8 cm’K mol-1for complex 2. The decrease in XmT withthe decrease in temperature should be due to the depopulationof Stark sublevels for Dy(m) or Tb(m). Fig.22Temperature dependent magnetic susceptibilities for complexes1 and 2. The red line represents the calculated values for complex 1. The magnetization dynamics of complexes 1 and 2 wereinvestigated under zero and in the presence of a dc filed. Inorder to find a suitable external field, the ac susceptibilities ofcomplex 1 were measured by varying the de fields from 0 to4000 Oe at 2 K. The results indicate that out-of-phase com-ponent x" values increase with the increase in temperature andreach a plateau (10 Hz) or a maximum (100 Hz) under theexternal dc field of 1500 Oe (Fig. S9, ESIt). Therefore, 2000 Oewas chosen for complex 1. Complex 2 shows no slow magneticrelaxation (Fig. S10, ESIt). Non-zero and frequency dependentx" values are observed under 2000 Oe in complex 1, but nopeaks in the x"vs. T plot appear down to 2 K (Fig. 3a). Thisphenomenon may be due to strong quantum tunnellingeffects of the Dy(m) ion and/or the fact that 2 K is too high toobserve the maxima of x". Although the barrier energy couldnot be determined by the Arrhenius law t=to exp(Uerr/kT), the-T0barrier energy is roughly evaluated using a previously reportedliterature method.59,60 The corresponding law is described asthe following equation: In(x"/x)=In(wto)+Ea/kpT. The data offrequencies 100 Hz, 316 Hz and 563 Hz are used to calculatethe energy barrier, affording the Ea value of about 7 K (Fig. 3b). We collected the reported six-coordinate Dy(m) complexes(Table 3)11,25,27-30,61-65 and analyzed their magnetic properttiessuccinctly. It can be seen from Table 3 that complexes withclose Dy-O/N/S/C bond distances generally have lower energybarriers than that of complexes with markedly different Dy-O/N/S/C bond lengths. To evaluate the effect of structural dis-tortion, we calculated the differences Ad (absolute values) ofthe average axial and equatorial coordination bond distanceswith the assumption that the larger differences should corres-pond to the greater structural distortion and uniaxial an- r-1K-1 Fig. 3 (a) Temperature dependence of the out-of-phase (") ac mag-netic susceptibilities for complex 1 under 2 kOe dc field with an oscil-lation of 10.0 Oe. (b) The ln(x"/x)-1/T plot. The red lines represent thefitting results. Table 3Magnetic data for six-coordinate Dy(mm) complexes Complexes" Dy-O/N/S/C (A) ^d(A) Deviation values Field (Oe) Ueff(K) Ref. [NisDy{(py)2C(H)O}](ClO4)3 2.257(2)-2.268(1) 0.00075 1.904 _ 28 Dy(BpMe)3 2.4762(15)-2.4860(15) 0.00025 15.802 11 Dy(HBPzMe2)s 2.450(3)-2.509(3) 0.00225 16.617 65 {(H3O)[Dy(NA)2}n 2.212( (3)-2.303((3 0.076 0.216 61 Complex 1 2.242( 5)-2.311((5) 0.026 3.791, 3.806 2000 7 This work [NizDyL2]ClO4 2.333( [5)-2.392(5) 0.0218 8.125 0 11 27 Dy(LoiPr)2](PF。) 2.246( 11)-2.264(12) 0.01 0.5226 750 13 29 NizDyL2]NO3 2.329( [2)-2.386(3) 0.0083 7.885 14 27 [Dy(L"QEt)2]](PF6) 2.250( 4)-2.256((4 0.002 1.661 600 26 29 Dy(Bc 2.572( (3)-2.582((3 0.001 15.606 1500 47 11 Zn2DyL2]NO3 2.183( [7)-2.389(7) 0.097 1.879 1200 64 30 Li(THF)44Dy4{N(SiMe3)2}4(u-SEt)(H4-SEt)] 2.222( [5)-3.0147(16) 0.175 3.029,4.119,4.688,4.794 0 66 62 Li(THF)4[IDyFc3Li2(THF)2 2.532( (5)-2.564(5) 0.0133 11.530,12.418 0 159 63 (L)Dy(N*)2] 2.296( [2)-2.4680(17) 0.135 18.321 0 190 64 DysO(OiPr)13] 1.951( [18)-2.66(2) 0.194 1.399, 1.947,3.318,2.696,3.744 0 528 31 [Dy4K2O(O'Bu)12] 2.068( (10)-2.515(10) 0.148 2.094,2.209,2.657,2.696 0 692 25 “HLa = tri(3-methoxysalicylidene)amino; HL = 2,2',2"-(((nitrilotris(ethane-2,1-diyl))-tris(azanediyl))tris(methylene))tris-(4-bromophenol);BpM]-= dihydrobis(methylpyrazolyl1)borate; [Be:`Me]-=dihydrobis(methylimidazolyl)borate); HNA=5-hydroxynicotinic acid;LH={N-[(2-MeO)C6HJ}N=C(Me)CH=C(Me)N(H){N'-([(2-MeO)C,Hs]}; HN*=HN(SiMe3)2. "The difference Ad of average axial coordination bond distances andaverage equatorial coordination bond distances.The degree of deviation from the octahedral calculated by the SHAPE software. isotropy. As expected, the result suggests that larger differencegenerallyaccompanies better magnetic performances.Complex [Dy4K20(O'Bu)12]C H14 with a high Ueff value of692 K has a Dy-Oaxial bond distance of 2.068(10) A which ismuch shorter than other Dy(m)-O bonds.Moreover, the mag-netic anisotropic axis of each dysprosium ion is coincidentwith the Oaxial-Dy(m)-Oalkoxide axis. Thus, six-coordinate Dy(m)complexes could exhibit excellent dynamic magnetism whenpossessing weak equatorial and strong axial coordinationdonors.6 The coincidence of the anisotropic axis and thecoordination principal axis is also important for SIMs.30,67Moreover, the existence of a sole principal axis is required forSIMs. For complex 1, no apparent exclusive principal axis ispresent, resulting in the small energy barrier. Magneticdilution is an effective strategy to remove the quantum tunnel-ling of single-molecule magnets and is also successful for six-coordinate Dy(m) complexes.25,29,61However, the magneticproperty of diluted sample {Dyo.05Yo.95} does not show theapparent distinctions from that of complex 1 (Fig. S11,ESIt). In order to understand the magnetic properties of complex1 theoretically, CASSCF calculations on the basis of X-ray deter-mined geometry (Fig. S12t for the complete structure used)were carried out with the MOLCAS 8.2 program package. Thecalculated DC magnetic susceptibilities are lower than theexperimental valuesiin the entire temperature range of2-300 K, but the XmT variation is coincident with the experi-mental data (Fig. 2). The difference between experimental andcalculated data may be due to the intrinsic errors caused bythe calculation method and magnetic measurements and thepresence of intermolecular magnetic coupling in complex 1,which is not considered in the calculations. Calculated energylevels (cm-1) of the Kramers doublets (KDs), g (gx, gy, gz)tensors and m values of the lowest KDs of the Dy" of complex1 are shown in Table S1 (ESIt). The local main magnetic axis of the ground Kramers doublet on Dyis not coincident withany Dy(m)-0 bond (Fig. S13, ESIt). Moreover, the calculatedeffective gz of 15.492 is much lower than 20 expected for a pure|±15/2Kramer doublet, and the ground-state wave function iscomposed of only 72%±15/2) Kramer doublet (72%|±15/2)+5%+7/2)+5%±3/2)+10%±1/2)), suggesting that the groundKDs are mixed largely by yDsyeveral m states, which leads to largeQTM in the ground state for complex 1 (Table S2, ESIt). Thecalculated tunneling gap between the two ground KDs (±15/2)is as high as 0.67 (Fig. 4), which also confirms the large QTMin the ground KDs for 1. Thus, an external DC magnetic fieldis required for the magnetization relaxation to be detected. 一 Fig.4 The calculated magnetization blocking barriers in complex 1.The thick black lines represent the Kramers doublets as a function oftheir magnetic moment along the magnetic axis. The green lines corres-pond to diagonal quantum tunneling of magnetization (QTM); the blueline represents off-diagonal relaxation process. The numbers at eacharrow stand for the mean absolute value of the corresponding matrixelement of transition magnetic moment. Fig. 55Fluorescence spectra of complex 2 in solid (red) and ethanolsolution (blue) (ex=295 nm). The energy gap between the lowest and ground-state Kramersdoublets is 35.1 cm(50.5 K), which is significantly largerthan the experimental energy barrier (Ea=7 K) under 2000 Oe.This significant difference may be ascribed to the fact thatCASSCF method does not include electron dynamic correlationeffect and the QTM cannot be fully suppressed by the externalDC magnetic field. Fluorescence The Tb(m) complexes usually show good fluorescence. Thefluorescence spectra in solid and methanolic solution forcomplex 2 with the excitation wavelength of 295 nm aredepicted in Fig. 5. The same characteristic emissions of 4f-4ftransitions for Tb(m) ions are observed,8 demonstrating thepresence of the Tb(m) molecules in the solution. Themaximum fluorescence emission (D4 →Fs) appears at545 nm, resulting in a green fluorescence. The energy transferfrom the ligand Lto Tb(m) ion owing to the antenna effect isresponsible for the characteristic fluorescence of complex 2. Interestingly, when we press or rub the powder of complex2 using a glass plate, the powder emits a brilliant green light(ESIt). This behavior is regarded as triboluminescence (TL)similar to that of the complex [TpPTb(CHCO2)2(H2O)].9 Inaddition, the TL colour is the same as that of photo-luminescence, which indicates that the mechanical rubbingoffers the energy required for fluorescence excitation. The TLphenomenon has been assumed to be related to the asym-metric or distorted local symmetry.70,71 The external pressureor friction force causes a molecular distortion and concomi-tant charge distribution of asymmetry, generating the excitedstate of the ligands L’ for fluorescence emission. The propel-ler-like molecules of complex 2 are apt to distort under exter-.nal pressure or friction, making it exhibit triboluminescence. Conclusions Two interesting chiral six-coordinated lanthanide complexes[Zn(L)Cl]sLn have been synthesized. The spontaneous resolu- tion gives two enantiomers of the chiral complexes. Complex 2shows strong green photo-fluorescence and tribolumines-cence. Magnetic measurements indicate that complex 1 dis-plays a field-induced slow magnetic relaxation, which is typicalof a single-ion magnet. A theoretical study proves that the tun-neling gap between two ground KDs (±15/2) is high, which isresponsible for the routine SIM properties of complex 1. Inaddition, studies on a magneto-structural correlation of six-coordinate Dy(m) complexes suggests that the axial structuraldistortion is a key factor for the observation of the SIM prop-erty.2 Thus, it is worthwhile to modulate the Dy-O bond distances to improve the magnetic properties of the present chiralsystem. This might be realized by the introduction of asym-metric ligands or mixed ligands. Future research along thisline is in progress in our laboratory. Conflicts of interest There are no conflicts to declare. Acknowledgements This study was supported by the 973 program (2013CB933403),the National Natural Science Foundation of China (Project No.91222104, 21571113), and the Natural Science Foundation ofJiangsu Province (BK20151542). Notes and references 1 N. Ishikawa, M. Sugita, T. Ishikawa, S. Koshihara andY. Kaizu, J. Am. Chem. Soc., 2003,125,8694. 2 S.-D. Jiang, B.-W. Wang, H.-L. Sun, Z.-M. Wang and S. Gao,J. Am. Chem. Soc.,2011, 133,4730. 3 F.-S. Guo and R. A. Layfield, Chem. Commun., 2017, 53,3130. 4 V. Chandrasekhar, S. Hossain, S. Das, S. Biswas andJ. P. Sutter, Inorg. Chem., 2013,52,6346. 5 X.-L. Li, J.-F. Wu, J.-K. Tang, B. L. Guennic, W. Shi andP. Cheng, Chem. Commun.,2016, 52,9570. 6 T. Pugh, V. Vieru, L. F. Chibotaru and R. A. Layfield, ChemSci., 2016, 7,2128. 7 S. K. Gupta,T Rajeshkumar,, GG.j.Rajaraman andR. Murugavel, Chem. Sci.,2016,7,5181. 8 P. Zhang, L. Zhang, C. Wang, S.-F. Xue, S.-Y. Lin andJ.-K. Tang, J. Am. Chem. Soc., 2014, 136,4484. ( 9 J. Xiong, H.-Y. Ding, Y.-S . Meng, C. Gao, X.J. Zhang,Z.-S. Meng, Y.-Q. Zhang, W . S hi, B . -W. W ang and S. Gao, Chem. Sci.,2017,8,1288. ) ( 10 W. J. Evans, M. A . A nsari a nd J. W . Ziller, Inorg. Chem.,1996,35,5435. ) ( 11 K. R. M eihaus, S. G. M i nasian, W. W . Lukens Jr. , S. A. Kozimor, D. K. Shun, T. Tyliszcak and J. R. Long,J. Am. Chem. S oc., 2014, 136,6056. ) ( 12 J . Liu , Y .-C. Chen, J.-L. L iu, V . V ieru, L . U n gur, J. - H. Ji a ,L.-F . Chibotaru , Y.-H . Lan, W. Wernsdorfer, S. G ao, X.-M. Chen a nd M.-L. Tong, J. Am. Chem. Soc., 2016 , 138, 5441. ) ( 13 F. Mori, T. Nyui, T. Shida, T. Nogami, K.-Y. Choi andH. Nojiri,J. Am. Chem. Soc.,2006, 1 28,1440. ) ( 14 S .-D. Jiang, B.-W. Wang, G. S u, Z .-M. W ang a n d S. Gao,Angew. C hem., I n t. E d., 2010, 122, 7610. ) ( 1 5 G . W . Rabe, J. R iede and A. S c hier, Inorg. C hem., 1996, 3 5 , 40. ) ( 16 D. Heitmann, C. Jones, D. P. M ills and A . S tasch, Dalton Trans.,2010,39,1877. ) ( 17 M. R en, Z .-L. Xu, S.-S. Bao, T.-T . Wang, Z.-H. Zheng, R. A. S. Ferreira , L.-M . Zheng and L . D. C arlos, DaltonTrans.,2016,45, 2974. ) ( 18 Y . B i, Y.-N. Guo, L. Z h ao, Y. Gu o , S.- Y . Lin, S.-D. Ji a ng,J . -K. Tang, B.-W. Wang and S . Gao, C h em.- E u r. J., 2011, 17,12476. ) ( 19 M.-J. Liu, K.-Q. Hu, C.-M. Liu, A. - L. Cui and H.-Z. Kou,Dalton Trans., 2017, 46,6544. ) ( 20 K .-Q. H u, X. J iang, S .-Q. W u, C.-M. Liu, A.-L. C ui a n d H.-Z. Kou, I norg. Chem., 2015, 54, 1 206. ) ( 21 E. M. F atila, M. Rouzieres, M. Jennings, A. J. Lough,R. Clerac and K. E. Preuss, J. Am. Chem. Soc., 2013, 135, 9596. ) ( 22 M. Tl.. B B enson, T. R. Cundari, L. C. SaundersS and S. O. Sommerer, Inorg. Chim. Acta, 1997,258, 127. ) ( 23 Y.-C. Chen, J.- L . L i u, L . Ungur, J. L i u, Q.-W. Li , L.- F . W a ng,Z.-P. Ni, L . F. Chibotaru , X.-M . Chen and M.-L. Tong, J. Am.Chem. Soc.,2016,138,2829. ) ( 24 Y.-S . D ing, N. F. Chilton, R. E. P.’ Winpenny andY.-Z. Zheng, Angew. Chem., Int. Ed., 2016, 55, 1 6071. ) ( 25 R. J. Blagg, L. Ungur, F. Tuna1, J. Speak, P. Comar,D. Collison, W. Wernsdorfer, E. J. L. McInnes, L . F . Chibotaruand R. E . P. Winpenny, Nat. C hem., 2 0 13,5, 6 7 3. ) ( 26 F.-S. Guo, B. M. Day, Y.-C. Tong, A. Mansikkamaki andR. A. Layfield, Angew. Chem., Int . Ed.,2017,56, 1 1445. ) ( 27 M.-X. Yao, Z.-X. Zhu, X .-Y. Lu, X.-W. Deng a n d S. Jing,Dalton T rans., 2016, 45, 10689. ) ( 28 C. G. Efthymiou, T. Stamatatos, C. Papatriantafyllopoulou, A. Tasiopoulos, W. Wernsdorfer, S. P. P erlepes a nd G. C h ristou, Inorg. Chem.,2010,49,9737. ) ( 29 K . S . Lim, J .J. Baldovi, S.-D. Jiang, B. H. Koo, D.-W. Kang,W. R . Lee, E. K . Koh, A. Gaita-Arino, E. C oronado,M. S lota, L. Bogani and C.-S. Hong, Inorg. Chem., 2017, 56, 4911 . ) ( 30 J .-L. Liu, Y.-C . Chen, Y.-Z. Z eng, W . -Q. Li n , L. Un g ur,W. W ernsdorfer, L . F . Chibotaru a n d M.-L. Tong, Ch e m. Sci., 2013, 4,3310. ) ( 31 R . J. Blagg, C . A . M uryn, E . J. L . M clnnes, F. Tuna a n d R. E. P. Winpenny,Angew. Chem., Int . Ed.,2011,50,6530. ) ( 32 C . Train, M. G ruselle and M . Verdaguer, Chem. Soc . Rev., 2011,40,3297. ) ( 33 Z .-G. Gu, C.-H. Zhan, J. Zhang and X.-H. Bu, Chem. S o c. Rev.,2016,45,3122. ) ( 34 H .-L. Qian, C.-X. Yang and X.-P. Yan, Nat. Commun., 2016, 7,12104. ) ( 35 J . L ong, J . R ouquette, J. M . T h ibaud, R. A. S. F er r eira,L. D. Carlos, B. Donnadieu, V . V ieru, L . F . Chibotaru,L. Konczewicz, J. H aines, Y. Guari a n d J. Larionova, Angew.Chem., Int. Ed.,2015, 54,2236. ) ( 36 E . C. Constable, G . Z hang, C. E. H ousecroft, M. N e uburger, S. Schaffner and W. D. Woggon, New J . C hem., 2009, 33, 1064. ) ( 37 E . Coronado, J. R. Galan-Mascaros, G. J. Gomez-Garcia andV.Laukhin, Nature, 2000,408, 447. ) ( 38 C.-M. Liu, R.-B. Xiong, D.-Q. Zhang and D. B. Zhu, J. Am Chem. Soc.,2010,132,4044. ) ( 39 A. Lennartson, M. Vestergren and M. Hakansson, Chem. - Eur.J.,2005,11,1757. ) ( 40 M. Hakansson. M. Vestergren, B. Gustafsson and G. Hilmersson,Angew. Chem., Int. Ed., 1999,38,2199. ) ( 41 J .-W. Zhang, J.-H. Luo, P.-M. Wang, B. D ing, Y.-C. Huang,.Z.-L. Zhao, J . Zhang and Y.-G. Wei, Inorg. Chem., 2015, 54 , 2551. ) ( 42 D .-P. L i, T.-W. Wang, C . -H. Li, D . -S. L i u, Y.-Z. Li a nd X.-Z. You, Chem. Commun.,2010, 46,2929. ) ( 43 S .-Y. Lin, C. Wang, L. Zhao,J. - F. Wu an d J.- K . Tang, DaltonTrans., 2015,44, 2 23. ) ( .44 E.-Q. Gao, S.-Q. Bai, Z.-M . Wang and C.-H. Yan, J. AmChem. Soc.,2003,125,4984. ) ( .45 E.-Q. Gao, Y.-F. Yue, S.-Q . Bai, Z. He and C.-H. Yan,J. AmChem. Soc., 2004, 126,1419. ) ( 46 R. Inglis, F. White, S.PPiligkos, W. Wernsdorfer, E. K. Brechin and G . S. P apaefstathiou, Chem. C ommun., 2011,47,3090. ) ( 47 D .-P. Li, X.-P. Zhang, T.-W. Wang, B.-B. M a, C.-H. Li, Y.-Z. LiandX.-Z. You, Chem. Commun., 2011,47,6867. ) ( 48 T. Shiga, K . . N Maruyama, G. N. Newton, R. Inglis, E. K. Brechin and H. Oshio, Inorg.Chem.,2014,53, 4 2 72. ) ( 49 Y.-L. Wang, L. Chen, C .-M. L iu, Z.-Y. Du, L.-L. Chen and Q.-Y. Liu, Dalton Trans.,2016,45,7768. ) ( 50 R. Anderes. M. Brissard. M. Gruselle. ,aC.. Train. J. Vaissermann, B. Malezieux. J.-P. Jamet and M. Verdaguer,Inorg. Chem.,2001, 40, 4633. ) ( 51 S. Mondal, S . M andal, L. C a rrella, A. J a na, M . F l eck,A. Kohn, E . Rentschler and S. Mohanta, Inorg. Chem.,2015, 54, 1 17 . ) ( 52 T..( Gupta,, G. V elmurugan, V. Rajeshkumar ir and G. Rajaraman,J. Chem.Sci., 2016 , 128,1615. ) ( 53 V. Pavlishchuk,K, F. Brikelbach. T. Weyhermuller,K. Wieghardt a nd P . Chaudhuri, Inorg. Chem., 2 002, 4 1,4405. ) ( 54 C. P . Sebli, S. E. Howson, G. J. C larkson a n d P. Scott,Dalton Trans., 2010,39, 4447. ) ( 55 E . Y. Tshuva, N. Gendeziuk and M. K o l, Tetrahedron Lett.,2001,42,6405. ) ( 56 G. K arlstrom, R. L indh, P . -A. Malmqvist, B. O . Ro o s, U. Ryde, V. Veryazov, P.-O. Widmark. M. .Cossi. B. Schimmelpfennig, P . N e ogrady an d L. S eijo, Co m put. Mater. Sci., 2003,28,222. ) ( 57 M. L lunell, D. Casanova J. C irera, P. A lemany and S.Alvarez, SHAPE, version 2.1, Barcelona, Spain, 2013. ) ( 58 M. B rissard, M. Gruselle, B. Maezieux, R . T houvenot,C. Guyard-Duhayon a n d O. Co n vert, Eur. J . I n org. Chem., 2001,1745. ) ( 59 S.-Y. Lin , L. Zhao, Y.-N. Guo, P. Zhang, Y. Guo a n d J .-K. Tang, Inorg. Chem., 2012,51,10522. ) ( 60 Y.-N. Guo, X.-H. Chen, S.-F. X ue a n d J.-K. T a ng, Inorg.Chem., 2011,50,9705. ) ( 61 B . N a, X.-J. Zhang, W. Shi, Y.-Q. Zhang, B.-W. Wang, C . Gao, S. Gao and P. Cheng, Chem. - Eur. J . ,2014, 20, 15975. ) ( 62 D . N . Woodruff, F. Tuna, M. Bodensteiner, R . E. P. Winpenny and R. A . Layfield, Organometallics, 2013,32,1224. ) ( 63 T. Latendresse, N. S . Bhuvanesh and M . Nippe, J. Am.Chem. S oc., 2017, 139,8058. ) ( 64 S .-S. Liu, Y.-S . Meng, Y.-Q. Zhang, Z.-S . Meng, K. L ang, Z. Liang, Z.-L . Zhu, C.-F. Shang , B.-W. Wang and S. Gao, Inorg. Chem., 2017,56,7320. ) 65 K. R. Meihaus, J. D. Rinehart and J. R. Long, Inorg. Chem.,2011, 50,8484. 66 N. F. Chilton, Inorg. Chem., 2015, 54,2097. 67 I. Oyarzabal,A. Rodriguez-Dieguez, M. Barquin,J.. M. Seco and E. Colacio, Dalton Trans., 2017, 46,4278. ( 68 Y .-M. L uo, S.-F. Li, J . Li, X.J. Chen and R.-R. Tang, J. RareEarths, 2010,28,671. ) ( 69 E . A. M ikhalyova, A. V. Yakovenko, N. Zeller, M. A . Kiskin, Y. V. Kolomzarov, I. L . Eremenko, A. W. Addison andV. V. Pavlishchuk, Inorg. Chem., 2015, 54,3125. ) 70 X.-R. Zeng, R.-G. Xiong, X.-Z. You and K.-K. Cheung, Inorg.Chem. Commun.,2000,3, 341. 71 J. Raush, V. Lorenz, C. G. Hrib, V. Frettloh, M. Adlung,C. Wickleder, L. Hilfert, P. G. Jones and F. T. Edelmann,Inorg. Chem., 2014, 53,1166. ( 72 D .-D. Yin, Q.Chen, Y.-S. Meng, H.-L. S un, Y .-Q. ZhangandS. Gao, Chem. Sci., 2015,6,3095. ) This journal is C The Royal Society of Chemistry alton Trans., alton Trans., his journal is C The Royal Society of Chemistry Two new chiral six-coordinate lanthanide complexes (1 for Dy and 2 for Tb) have been synthesized usingthe achiral flexible ligand H 2 L (2,2’-[1,2-ethanediylbis[(methylimino)methylene]]bis[4,5-dimethylphenol]).Both complexes crystallize in the chiral P1 group space, and the enantiomers [Zn(L)Cl] 3 Dy·MeOH·0.5H 2 O(Λ-1), [Zn(L)Cl] 3 Dy·1.5H 2 O (Δ-1), [Zn(L)Cl] 3 Tb·1.5H 2 O (Λ-2) and [Zn(L)Cl] 3 Tb·H 2 O (Δ-2) are isolated in thereaction. Three [Zn(L)Cl] − anions coordinate to the central lanthanide ion using two phenoxo oxygenatoms of L 2− , and the lanthanide ion has the coordination geometry of D 3 . Complex 1 exhibits a field-induced slow magnetization relaxation, which is typical of a single-ion magnet (SIM). Ab initio calculationson complex 1 and studies on magneto-structural correlationship of six-coordinate Dy( III ) SIMs indicatethat the small energy barrier of complex 1 might be related to the absence of a unique anisotropic axis forthe regular octahedral coordination configuration of Dy( III ) and the high quantum tunneling gap betweenthe ground states (m J = ±15/2). Complex 2 displays green photofluorescence and triboluminescence.
关闭-
1/8

-
2/8
还剩6页未读,是否继续阅读?
继续免费阅读全文产品配置单
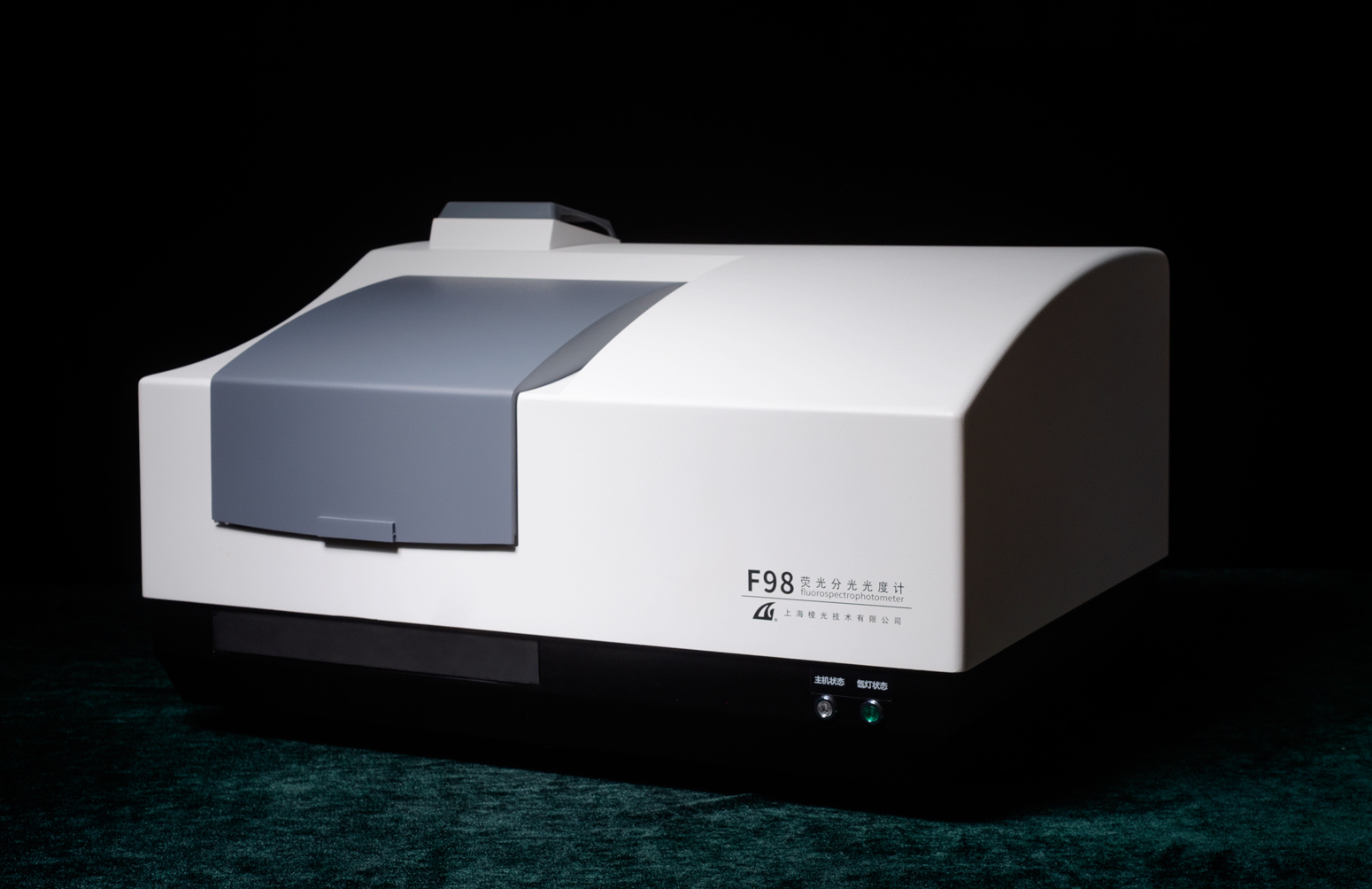
上海棱光技术有限公司为您提供《Dy( III ) and Tb( III )中structure, fluorescence, and magnetism?检测方案 》,该方案主要用于Dy( III ) and Tb( III )中structure, fluorescence, and magnetism?检测,参考标准《暂无》,《Dy( III ) and Tb( III )中structure, fluorescence, and magnetism?检测方案 》用到的仪器有棱光技术F98荧光分光光度计(2017)。
我要纠错
推荐专场
相关方案



 咨询
咨询




