方案详情文
智能文字提取功能测试中
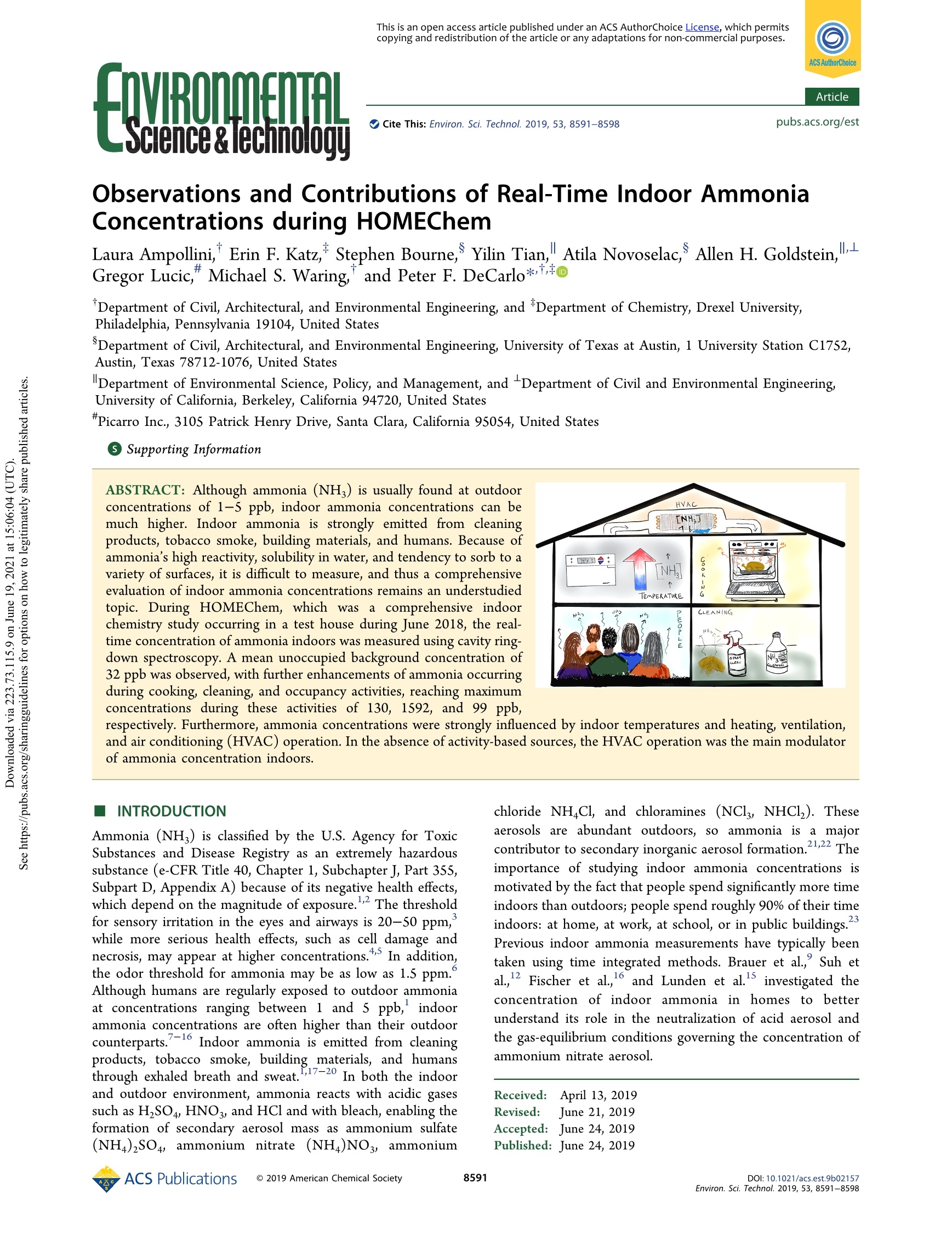
This is an open access article published under an ACS AuthorChoice License, which permitscopying and redistribution of the article or any adaptations for non-commercial purposes.MROLETALScence& ecTo OOTi Cite This: Environ. Sci. Technol.2019,53,8591-8598 ArticleEnvironmental Science & Technology Observations and Contributions of Real-Time Indoor AmmoniaConcentrations during HOMEChem Laura Ampollini, Erin F. Katz, Stephen Bourne, Yilin Tian, Atila Novoselac, Allen H. Goldstein,Gregor Lucic, Michael S. Waring, and Peter F. DeCarlo*o Department of Civil, Architectural, and Environmental Engineering, and *Department of Chemistry, Drexel University,Philadelphia, Pennsylvania 19104, United States SDepartment of Civil, Architectural, and Environmental Engineering, University of Texas at Austin, 1 University Station C1752,Austin, Texas 78712-1076, United States Department of Environmental Science, Policy, and Management, and -Department of Civil and Environmental Engineering,University of California, Berkeley, California 94720, United States "Picarro Inc., 3105 Patrick Henry Drive, Santa Clara, California 95054, United States Supporting Information ABSTRACT: Although ammonia (NH) is usually found at outdoorconcentrations of 1-5 ppb, indoor ammonia concentrations can bemuch higher. Indoor ammonia is strongly emitted from cleaningproducts, tobacco smoke, building materials, and humans. Because ofammonia's high reactivity, solubility in water, and tendency to sorb to avariety of surfaces, it is difficult to measure, and thus a comprehensiveevaluation of indoor ammonia concentrations remains an understudiedtopic. During HOMEChem, which was a comprehensive indoorchemistry study occurring in a test house during June 2018, the real-time concentration of ammonia indoors was measured using cavity ring-down spectroscopy. A mean unoccupied background concentration of32 ppb was observed, with further enhancements of ammonia occurringduring cooking, cleaning, and occupancy activities, reaching maximumconcentrations during these activities of 130, 1592, and 99PPb, respectively. Furthermore, ammonia concentrations were strongly influenced by indoor temperatures and heating, ventilation,and air conditioning (HVAC) operation. In the absence of activity-based sources, the HVAC operation was the main modulatorof ammonia concentration indoors. INTRODUCTION Ammonia (NH) is classified by the U.S. Agency for ToxicSubstances and Disease Registry as an extremely hazardoussubstance (e-CFR Title 40, Chapter 1, Subchapter J, Part 355,Subpart D, Appendix A) because of its negative health effects,which depend on the magnitude of exposure. The thresholdfor sensory irritation in the eyes and airways is 20-50 ppm,while more serious health effects, such as cell damage andnecrosis, may appear at higher concentrations.5 In addition,the odor threshold for ammonia may be as low as 1.5 ppm.Although humans are regularly exposed to outdoor ammoniaat concentrations ranging between 1 and s ppb, indoorammonia concentrations are often higher than their outdoorcounterparts.7-16 Indoor ammonia is emitted from cleaningproducts, tobacco smoke, building materials, and humansthrough exhaled breath and sweat.,17-20 In both the indoorand outdoor environment, ammonia reacts with acidic gasessuch as HSO4, HNO3, and HCl and with bleach, enabling theformation of secondary aerosol mass as ammonium sulfate(NH4)2SO4, ammonium nitrate(NH)NO, ammonium chloride NHCl, and chloramines (NCl, NHCl,). Theseaerosols are abundant outdoors, so ammonia is a majorcontributor to secondary inorganic aerosol formation.21,22 Theimportance of studying indoor ammonia concentrations ismotivated by the fact that people spend significantly more timeindoors than outdoors; people spend roughly 90% of their timeindoors: at home, at work, at school, or in public buildings.Previous indoor ammonia measurements have typically beentaken using time integrated methods. Brauer et al., Suh etal., Fischer et al.,and Lunden et al.investigated theconcentration of indoor ammonia in homes to betterunderstand its role in the neutralization of acid aerosol andthe gas-equilibrium conditions governing the concentration ofammonium nitrate aerosol. ( Received: A pril 1 3, 2019 ) ( Rev i sed : J une 21, 2019 ) ( Accep te d:1 : June 24, 2019 ) ( Published: J une 24, 2019 ) Leaderer et al. 4 investigated how indoor concentrations ofammonia changed in homes with and without kerosene heatersduring winter months, and with and without air conditioningduring summer months. Atkins and Lee, Tidy and Cape,10Tuomainen et al., and Jarnstrom et al. studied indoorconcentrations in different rooms of homes and new buildingsbefore and after the first months of occupancy. The ammonia17concentration in office buildings was the focus of Sisovic et al.and Salonen et al.2 while Li and Harrison, Gomzi,3 andMeininghaus et al. measured ammonia concentrations atuniversities and schools. Previous studies were often completed using diffusivesamplers or active samplers with denuders, methods whichlead to numerous limitations, such as lower accuracy andnonreal time measurements.Hence, there is a need formeasurements indoors with improved accuracy and high timeresolution so that factors that influence dynamic ammoniaconcentrations indoors can be determined. We present hereinadetailed assessment of indoor ammonia concentrationsmeasured in a test house using the method of cavity ring-downspectroscopy, allowing us to explore the impact of commonactivities such as cleaning, cooking, and occupancy on indoorammonia concentration dynamics, as well as the impact of aheating, ventilation, and air-conditioning (HVAC) system. MATERIALS AND METHODS HOMEChem. The measurements presented in this paperwere conducted during the HOMEChem experiment whichtook place at the University of Texas at Austin over 4 weeks inJune 2018. The goal of HOMEChem was to perform avariety of activities and experiments within the UTest house, athree bedroom and two-bath manufactured home with a floorarea of 110 m and a volume of 250 m,described in detail in astudy by Novoselac and Siegel, and to measure concen-trations of a number of gases and aerosols, leading to ananalysis of the everyday chemistry in indoor domesticenvironments. During the 4-week experiment, factors such as temperature,relative humidity (RH), HVAC system parameters, andnumber of people in the house, in addition to logging specificactivities performed and products used, were recorded using avariety of instruments and methods. The activities conductedduring HOMEChem can be placed into three macrocategorieswhich reflect common everyday indoor activities: cleaning,cooking, and occupancy. Although different types of subexperi-ments were conducted over the entire month of HOMEChem,only the ones most affecting ammonia concentration will be. considered and described in this study. Instrumentation. Ammonia concentrations were meas-ured with a Picarro G2103 Analyzer. This instrument uses thecavity ring-down spectroscopy (CRDS) analytical technique toprovide real-time measurement of ammonia at parts per billion(ppb) levels. We report 30 s average concentrations, althoughhigher time resolution is possible. Measurement accuracy is 0.5ppb ± 5% of reading with a precision of 0.10 ppb +0.1% ofreading for 30 s of data. The instrument’s molar absorptivity,or extinction coefficient, was calibrated at the Picarro factory inSanta Clara, CA, by comparison to a Calibration StandardAnalyzer immediately prior to shipping to Drexel University.The calibration of this Calibration Standard Analyzer wasvalidated by the National Physical Laboratory (UnitedKingdom) using gravimetric standards and reported to bewithin 1% of the standard, with an expanded uncertainty of 2%.28 Using the Calibration Standard Analyzer approach andsupporting studies of CRDS stability over time (0.1% slopechange/year),only periodic validation using a nonreactivegas (CO,) is required to validate the calibration. The Picarro G2103 Analyzer was placed in the kitchen of theUTest house, above the refrigerator ataa height ofapproximately 2 m and a distance of approximately 4 mfrom the stoveand oven. The inlet of the instrument was short,at approximately 5 cm long, with an inline Teflon filter toprevent particle intrusion into the instrument. Although thereis a reported carbon dioxide (CO) measurement by theG2103, we instead use the CO, measurement from the PicarroG2401 instrument (as described in ref 32). Temperature andRH data were recorded by a data logger (HOBO Data Logger,model U12-012, Onset Computer Corp., Bourne, MA) placednext to the Picarro, with 1 min resolution The total air exchange rate through the HVAC system wasmeasured to be 7.9±0.6 h-by summing the total flow fromsupply registers in the home. Air flow through the HVAC wasconstant, regardless of air-conditioning demand (HVAC fanwas always on). Measurements of HVAC airflow were madeusing a Shortridge Instruments CFM-88L flow hood with anaccuracy of ±3% of reading +7 ft /min. No HVAC filters wereinstalled in the system so as to prevent their influence on theexperiments. The fresh air system air exchange rate wasapproximately 0.5 h- unless specified otherwise, and wascontinuously monitored using an IEbtron Advantage IIIHTx104-PE with an accuracy of ±3% for the range of flowrates measured. Because of high expected latent loads in thetest house, a secondary dehumidifier was always active in theHVAC air handling unit. Temperature of the supply and return airflows weremeasured using Omega Instruments model 44033 thermistors,connected to GW Instruments iNet-100 data acquisitionhardware using Omega OMX-R4.7K precision shunt resistors.This combination provided an accuracy of ±0.1C in therange of 0-70°C. RH probes were located inside the HVACsupply diffuser, at the HVAC return register, and in the kitchenarea of the home. The RH was measured using Veris modelHD2XVSX humidity sensors with an accuracy of ±2% from 10to 80% RH. The window and door open/closed status was monitored bywireless sensors (SmartThings, Inc., Mountain View, CA,USA), with sensor arrangement reported in the studyoverview.9 Activities and Experiments. Background Measurement.One 12-h daytime background measurement was taken whenno people and no activities were taking place in the UTesthouse. This background measurement was recorded on June15, 2018, from 8:00 a.m. to 8:00 p.m. During this period, theair-conditioning (AC) thermostat set-point temperature wasset at 76 °F (~25.5°C). Nighttime High Temperature. To study the relationship ofhow chemical emissions from species such as ammoniarespond to temperature, measurements from the night ofJune 24, 2018, to the morning of June 25, 2018, were recordedas the set-point temperature in the UTest house was set at 90°F (~32.2 ℃). Limited activity took place during these hours;in particular, only a nighttime ozone experiment was beingperformed with two people entering the house at 9:55 p.m. and2:55 a.m. Response Time Activity. The "response time” activity wasperformed on June 4, 2018, with the goal of determining the time taken by a variety of measured chemical species withinthe UTest house to reach a steady-state condition after thedoor and windows of the house were sequentially opened andclosed. During the response time activity, the air conditioningwas turned off at 10:00 a.m. and the door and windows wereopened and closed at 30 min intervals until 8:30 p.m. The airconditioning was turned on at 8:35 p.m., signifying the end ofthe activity. Cleaning Experiment. The cleaning experiment wasconducted on June 26, 2018, and consisted of a combinationof mopping the floor with vinegar and spraying ammonia onother surfaces such as windows, the table, and the stove. Thecleaning experiment occurred in the living room, adjacent tothe kitchen. The ammonia spray used consisted of a solution ofHDX 64 oz. Lemon Ammonia (1-3 wt % of ammonia) thatwas mixed in the spray bottle according to manufacturer’sinstructions. The house was cleaned at three different timesduring the day. The experiment was conducted as follows: at12:35 p.m., floors are mopped with vinegar for 10 min, with a 1min break; surfaces are sprayed with ammonia solution for Smin; at 4:35 p.m., surfaces are sprayed with ammonia solutionfor S min, with a 5 min break; floors are mopped with vinegarfor 10 min; at 8:08 p.m., floors are mopped with vinegar for 10min, with a 10 min break; surfaces are sprayed with ammoniasolution for 5 min. The starting and ending weights of cleaningsolutions can be found in Table S1, Supporting Information. Cooking Experiment. The cooking experiment wasconducted on June 18, 2018, and consisted of the preparationof an"American Thanksgiving-style"dinner menu consisting ofturkey, roasted brussels sprouts with pancetta, smashed sweetpotatoes with marshmallows, cranberry sauce, and stuffing.Cooking began at 10:00 a.m. and ended at around 3:30 p.m.,with the dinner taking place between 4:00 and 5:00 p.m. Occupancy. Ammonia concentrations were measuredduring the HOMEChem Open House, an event that tookplace on June 22, 2018, which opened the UTest house tovisitors including scientists, scholars, and industry partnersfrom around the country. The occupancy in the house variedgreatly throughout the day. The open house schedule consistedof three tours and the recording of a radio program. Tourstook place 9:30-10:45 a.m., 12:00-1:00 p.m., and 1:00-2:00p.m. Because this event was not considered a subexperiment,precise data on the number of occupants throughout the dayare not available. However, approximate values based on visualobservation were recorded and are presented in the resultsbelow. CO, levels also provide an estimate of occupancy. Data Analysis. Collected data from all instruments wereanalyzed using Igor Pro 7 (Wavemetrics, Lake Oswego, OR).Ammonia concentrations were investigated in the context ofother measured parameters including temperature and RH ofthe house and HVAC supply air. In addition, other informationcollected in the activity log of the HOMEChem experiment,such as the periods when windows and doors were open, wereintegrated into the analysis to give a more comprehensive viewof the state of the house. In all experiments,a categorization ofthe cooling coil state was required. We categorize the coolingcoil as ON, meaning that its surface was wet, when the supplyair temperature was less than 20 °C, with the only exceptionbeing the night of June 25, 2018, when the air conditioning(AC) was set at a higher temperature than usual and thus atemperature of 27 °C was used as the cooling coil threshold.These temperature values were determined graphically byexploring the inverse relationship between HVAC supply air relative humidity and temperature. When the air conditioningis ON and thus the cooling coil as well, the temperature of thesupply air decreases rapidly while the humidity of the supplyair increases until the cooling coil is OFF again. RESULTS NH Concentration Values. Figure 1 summarizes theammonia concentrations observed in previous studies, with NH3 (ppb) Figure 1. Indoor NH, concentrations found in previous studiescompared to the frequency of indoor NH, concentrations foundduring the HOMEChem experiment (indicated by the yellowhistogram). The concentration values are classified by taking intoaccount the season in whicchh 1the measurements were conducted(summer JJA or winter DJF) and location (schools, offices, andhomes) when specified. additional details on the studies given in Table S1. Theconcentration values are classified according to the season inwhich the measurements were conducted (when specified) andthe location of the studies (schools, offices, or homes). Allvalues but one are greater than 10 ppb, a level that is33considered a high outdoor concentration of ammonia.Schools and offices were locations with the highest ammoniaconcentrations, reaching maximums of 440 and 103 ppb,respectively. Ammonia in homes ranged between 8.1 and 67.7ppb. The concentrations of ammonia found in homes arecompared with the concentrations found during HOMEChemindicated by the histogram. The histogram seems to be in linewith the previous results and provides additional informationon the frequency of concentration values. Values for the minimum, 10th percentile, mean, 90thpercentile, and maximum NH, concentration measured duringthe different activities described above are summarized inTable 1. These activities include the background measurement,nighttime high temperature, response time, cooking activity,cleaning activity, and occupancy. The background concen-tration had a mean of 31.9 ppb and each activity caused theammonia concentration to rise. These summary values reflectthe range of measurement, but do not provide insight into thehigh time resolution dynamic changes in ammonia concen-tration. NH, Concentration Dependence on Temperature.Background Measurement and Nighttime High Temper-ature. Indoor ammonia concentrations show a strongdependence on the indoor temperature at the UTest house.Figure 2 shows the temperature, RH, and ammoniaconcentration for two different days. Figure 2a shows theunoccupied background measurement with the house main- activity classification min 10th % mean 90th % max min 10th % mean 90th% max min 10th% mean 90th % max background 24.2 26.3 31.9 38 40.6 24.5 24.8 25.3 25.8 26.1 53.2 54 57.8 61.7 63.2 nighttime high temp 40 57.5 65.4 72.7 74.3 29.3 32.3 32.7 33.3 33.5 46 47.3 49.1 50.6 51.6 response time 20 28.5 52.5 80 91.6 24.8 27.1 31.2 34.9 35.5 45.8 47 53.2 60 69.5 cooking 23.8 30 62.1 102.4 130.1 25.4 26 27.8 30.2 30.7 48.9 50.1 52.1 53.9 62.7 cleaning 19.1 26.1 72.9 94.9 1592 24.1 25 26.7 29.9 35.3 44.6 48.5 52.7 58.8 72.7 occupancy 29.6 34.9 51.6 86 99 24.7 25.4 26.4 27.6 27.8 49.9 51.9 56 60.1 67.7 “Summary of minimum, 10th percentile, mean, 90th percentile, and maximum NH, concentration, temperature, and relative humidity measuredduring the unoccupied background measurement and other activities performed during HOMEChem. Figure 2. NH, concentration, temperature, relative humidity, and cooling coil on/off during unoccupied background on June 15, 2018, with AC setat 76 ℉(~25.5℃) and nighttime high temperature on June 24-25, 2018, with AC set at 90 °F (~32.2C). Figure 3. NH, concentration, temperature, and relative humidity during response time activity. The yellow columns show the 30 min house flushperiods, that is, the time range when door and windows were opened. taining a mean temperature of 25.3 °C and relative humidity of57.8%. For these conditions the mean ammonia concentrationis 31.9 ppb. Figure 2b shows the measurements during thenighttime high-temperature period where indoor temperaturesaveraged 32.7 C with RH at 49.1% and a mean ammoniaconcentration of 65.4 ppb. The average temperature during thenighttime high-temperature measurement was 7.4 °C hotterthan that during the background measurement,whicheffectively doubled the indoor ammonia, increasing theconcentration by 33.5 ppb. These levels of ammonia aresimilar to those in Leaderer et al.4 for homes in the case ofsummer with AC on (Table S1). Although the mean ammonia concentrations during the 2days are notably different, with concentrations ~36 ppb higher on average at the elevated temperatures, they display the samefine-scale temperature-dependent behavior, with increases anddecreases in ammonia concentration over short time scalesbased on fluctuations in indoor temperature and HVACoperation and presence of water on the cooling coil. Thisbehavior is consistent with observations of cyclic uptake ofwater-soluble organic species in a residential HVAC systemoperating on a duty cycle.For the background measurement,ammonia concentration varied from a minimum of 24.2 ppbtoa maximum of 40.6 ppb, matching the rise and fall oftemperature, which varied from 24.5 to 26.1 C. For thenighttime high-temperature measurement, ammonia concen-tration varied from a minimum of 40 ppb to a maximum of 74.3 ppb, matching the rise and fall of the higher temperatures,from 29.3 to 33.5 °C. In Figure 2, the behavior of the HVAC system and its effecton ammonia concentration can be easily1 yo cbserved: when AC isON, and thus the cooling coil is wet (because of thetemperature of the cooling coil being lower than the dew-pointtemperature of the air), the temperature, the RH, and theammonia concentration drop until AC is switched OFF, atwhich point all three immediately start to rise until AC isswitched ON once again; this pattern continues while no otheractivity is taking place within the house. This is alsohighlighted by the final time period represented in Figure 2(7:07 a.m. on June 25, 2018), when the AC is returned to thelower temperature of 76 °F, and temperature and ammoniaconcentration return to background measurement levels by10:00 a.m. The HVAC fan was always ON in the test house(even when the cooling coil was OFF), which is not the case ina normal residential HVAC system; this could have enhancedthe re-evaporation of NH, into the supply air when the coilingcoil turns OFF. Response Time. Figure 3 shows NH, concentration,temperature, and RH during the response time activity. At10:00 a.m. the AC was turned off and the temperature steadilyincreased throughout the day from a starting temperature of24.8 °C to a final maximum temperature of 35.5°C. Ammoniaconcentrations also increased throughout the day, with ahigher starting concentration for each sequential recoveryexperiment. The maximum ammonia concentration reachedduring the day was 91.6 ppb, but this concentration droppedrapidly during the house flush periods when doors andwindows were opened to rapidly ventilate the house withoutdoor air with low ammonia concentrations. Note that theammonia concentrations measured indoors never dropped totypical outdoor values, indicating a continuous emission ofindoor ammonia. Because concentrations of ammoniaremained high during all the windows and doors beingopened, this suggests a large source of ammonia on indoorsurfaces which can rapidly be liberated into the gas phase tomaintain equilibrium. This surface-gas equilibrium mechanismis similar to the proposed HONO equilibrium mechanismdescribed in detail by Collins et al. This maximum ammoniaconcentration corresponded with the maximum temperaturepoint and was almost 3 times higher than the room-temperature background measurement and roughly 18 timesgreater than a typical moderate-high outdoor concentration of5 ppb, suggesting that a house without air conditioning mayreach ammonia concentrations that are quite high during warmperiods. Figure 4 utilizes data from Figures 2 and 3 and plots thenatural logarithm of ammonia concentration versus the inverseof the temperature (in K) using the six peaks of NH,concentrations recorded during the response time activity,plus the average off peak concentrations from the twobackground measurements. The linearity of this plot(R=0.97) suggests one of two potential mechanisms occurringsimultaneously or alone: (1) ammonia emissions from buildingmaterials respond to temperature in an exponential fashion,(2) standard sorption kinetics are governing ammonia-surfacebehavior. The relationship fit in Figure 4 was used to predictbackground ammonia concentration as a function of temper-ature during the cooking, cleaning, and occupancy experi-ments, to better quantify and visualize the ammonia emissionsobserved from these activities. Note that this relationship does Figure 4. Plot of the natural logarithm of ammonia concentrationversus inverse of temperature (in K). not hold when doors and windows are open due to dilutionwith low NH, concentration and warmer outdoor air. Cooking Experiment. The ammonia concentration duringthe simulated Thanksgiving dinner activity is shown in FigureSa. During this activity, ammonia concentration ranges from23.8 to 130.1 ppb, with a mean concentration value of 62.1ppb. The temperature throughout this activity remainsPPrelatively stable, with a mean temperature of 27.8C. Thistemperature is slightly higher than the set temperature on thethermostat(76 °F=24.4C) because of the cooking activitiesand associated thermal load. When the measured temperature is used, the relationship inFigure 4 was used to estimate the background ammoniaconcentration, demonstrating that house emissions alone donot account for the enhancements in ammonia concentrationsobserved during the cooking activity. Thus, cooking emissionsare an important source of ammonia here, and these emissionshave a larger effect on the ammonia concentration than thetemperature-based background emissions on this day. In Figure Sa, ammonia concentration shows a rapid increaseeach time the oven is opened. In fact, the first five peaks on thegraph (labeled as 1, 2, 3, S, and 7) correspond with openings ofthe oven door associated with turkey and pancetta beingcooked inside the oven. This relationship between the openingof the oven door and peaks in ammonia levels suggests that thethermal decomposition of amino acids in proteins of the meatmay be an important source of ammonia during cookingactivities.36 In contrast, stir-frying of vegetables was donefrequently during lHOMEChem and did not show theammonia enhancements (Figure S1) observed from roastingturkey, supporting the amino acid breakdown pathway as thelikely source of ammonia emission. Cleaning Experiment. During the ammonia-based productcleaning activity, the maximum ammonia concentrations of theentire HOMEChem experiment are reached. As shown inFigure Sb, there are three main peaks that follow the threecleaning procedures described in the Materials and Methodssection. Peaks 1 (976 ppb) and 3 (732 ppb) result fromammonia being sprayed on surfaces after the floors aremopped with vinegar. The second peak, 1592 ppb, which is themaximum ammonia concentration value recorded over theentire HOMEChem experiment, results after ammonia issprayed before mopping the floors with vinegar. The alternating order of vinegar mopping and ammonia-product spraying, with consequent lower concentrations ofammonia when spraying occurred after mopping, suggests thatthe neutralization reaction between ammonia and acetic acidhappens quickly, limiting ammonia concentrations fromclimbing to even higher values by removing some fraction ofthe ammonia when it is added to the vinegar-coated floor. In Figure 5. NH concentration, NHs predicted, temperature, relative humidity, and cooling coil on/off during the cooking activity (Thanksgivingdinner) and the cleaning activity (combination of mopping the floor with vinegar and spraying ammonia on surfaces). Part (a)1,2,3: Open oven tobaste turkey; 4: begin frying turkey on stove; 5: turkey out of oven; 6: pancetta into oven; 7: pancetta out of oven; 8: start cleanup. Part (b) 1,3:NHs spray starts (after mopping); 2: NH; spray starts (before mopping). Time intervals when windows/door and windows were opened areindicated with"D+W open"and"W open". NH, decay due to pure air exchange loss is shown in graph B. Figure 6. Graph (A) NH, concentration, NHs predicted, temperature, relative humidity, and cooling coil on/off during the occupancy activity(Open House). Time intervals when the door was open are indicated by the black trace. The CO2 gaps show time intervals when the outdoorconcentration was measured, while outdoor CO,indicates the mean outdoor CO2 collected over the time period shown in the figure. Graph (B)Fitting lines and ANH (NH;-NHs predicted) versus ACOz (COz- CO outdoor) during each tour of the open house. Slopes from fit: Tour 1:0.015 ppb for ppm of CO; Tour 2: 0.030 ppb of NH, for ppm of CO2; Tour 3: 0.048 ppb of NH, for ppm of CO. all cases, though, the highest indoor concentrations ofammonia were observed with values on the order of 1 ppmfor each cleaning event. After each peak, which occurred during the spraying ofammonia during cleaning, ammonia then returned to lowerlevels within 1-3 h. After the peak ammonia concentrations,indoor ammonia levels returned to 60 ppb after 44, 62, and 37min for cleaning activities 1, 2, and 3, respectively. The pure airexchange traces in Figure Sb show the decay of ammonia ifcaused only by the air exchange rate. In this case, the ammoniaconcentration would return to 40 ppb after each peak in muchlonger time periods. The steep decline of ammonia observedafter each peak could be caused by the indoor uptake fromchemical reactions and/or the solubility in the water film onthe surfaces in the house and in particular on the active coolingcoil and dehumidifier. Further investigation is needed to betterunderstand the uptake dynamics of ammonia in the indoorenvironment. Note that predicted values of NH, based on temperature are no longer valid when ventilation with outsideair occurs until equilibrium can be re-established after closingdoors and windows. Occupancy. The higher occupancy that occurred during theOpen House was correlated with higher ammonia concen-trations, reaching a peak of 99 ppb, as seen in Figure 6a. It wasestimated by visual observation that 15-20 people were in thehouse during both the first and the second tour (9:30-10:45a.m. and 12:00-1:00 p.m.), while a higher number of around20-25 people were in the house during the third tour (1:00-2:00 p.m.). This higher number of people and the consecutiveoccurrences of the second and third tours may contribute toexplaining why ammonia levels reach a maximum after thethird tour. This can also be seen by CO, levels, which show anincrease in CO, concentration from 1275 to 2241 ppm overthe first tour and from 1800 to 3010 ppm over the second andthe third tour. Figure 6b shows the incremental change ofammonia as a function of the increment of CO, due to the different number of people taking part in each tour of the openhouse. While ammonia concentration points are welldistributed over the fitting line in the first tour, suggesting asteady increase of ammonia with occupancy, this behavior isnot visible over the following two tours. A possible explanationof this trend is that the loss of ammonia due to the solubility inthe water film of the coils and/or other surfaces in the housereach saturation and are not as effective at taking up additionalammonia. As part of the HOMEChem project, there werededicated days to varying the number of occupants in the testhouse; however, changes in ammonia concentration were smallcompared to changes from HVAC operation, as can be seen inFigure S2. Quantifying ammonia emission from humansremains a challenge and further studies are requiredtcunderstand this topic. The mean ammonia concentration throughout the OpenHouse day is 51.6 ppb, while the mean temperature during theOpen House is 26.4 C, a value typical for the averagetemperature during the background measurements. During theOpen House, however, the AC was running constantly to keepthe temperature low because of a combination of factors,including the number of people inside the house, theuncontrolled opening and closing of the door, and the highoutside temperature. These observed ammonia concentrationsare consistent with studies by Sisovic et al. and Gomzi,which found an indoor ammonia concentration in the summer,in offices and schools, respectively (i.e., places with highoccupancy), of more than 100 ppb. Ammonia concentrations measured during the HOME-Chem experiment showed significant enhancements of indoorammonia concentration compared to typical outdoor values.The values observed were consistent with other studies ofindoor ammonia concentration; however, we suggest addi-tional measurements across climate zones and in differentseasons are needed to assess how applicable the observedindoor levels of ammonia are across other indoor environ-ments and HVAC operating conditions. Overall, indoorconcentrations of ammonia appear to be strongly controlledby gas-surface equilibrium similar to observations forHONO.35工These concentrations are modulated by theHVAC system and the cooling coil state. The higher gas-phase concentrations of ammonia indoors may be importantdrivers for chemical reactions as compared to outdoor levels.Reactions in which ammonia is the limiting reagent will beenhanced up with the higher concentrations indoors. Thepresence of ammonia may also serve as an avenue for acidicgases (e.g., HCl, HNO3) to react and partition into the particlephase as ammonium salts. Furthermore, because of the highsolubility of ammonia in water, and the wet surfaces in theHVAC system when the cooling coil is on or other wet surfacesindoors, there may be avenues for aqueous chemistry to occurand/or the HVAC system may be a sink for ammonia. Thiswould follow the mechanism of uptake of water-solubleorganic species as described by Duncan et al. 4 Theseobservations of ammonia concentrations during HOMEChemare the first, to our knowledge, of high time resolution indoorammonia measurements with the ability to clearly identifydifferent sources and processes impacting ammonia concen-trations in the indoor environment. ASSOCIATED CONTENT ⑤ Supporting Information The Supporting Information is available free of charge on theACS Publications website at DOI: 10.1021/acs.est.9b02157. Table of details for previous integrated measurements ofindoor ammonia; additional figures showing othercooking and occupancy measurements (PDF) AUTHOR INFORMATION Corresponding Author *Phone: (215)895-2345; fax: (215)895-1363; e-mail: pfd33@drexel.edu. P.F.D.: Department of Civil, Architectural andEnvironmental Engineering, Drexel University, Philadelphia,PA 19104, USA. ORCID D Peter F. DeCarlo: 0000-0001-6385-7149 Notes The authors declare no competing financial interest. ACKNOWLEDGMENTS We thank Picarro Inc. for the loan of the G2103 analyzer foruse during HOMEChem. This work was funded by grantsfrom the Alfred P. Sloan Foundation Chemistry of IndoorEnvironments Program (G-2017-9944 and 2016-7050) andDrexel University Steinbright co-op funding. We acknowledgethe University of Texas at Austin for hosting HOMEChem andMarina Vance and Delphine Farmer for leading the project. REFERENCES (1) Roney, N.; Llados, F. Toxicological profile for ammonia. https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=11=2.2004. ( (2) S wotinsky, R. B.; C hase, K . H . He a lth ef f ects of exposure toammonia: scant i n formation. Am. J. Ind. Med. 1990, 17 (4),515-21. ) (3) Nielsen, G. D.; Wolkoff, P.; Alarie, Y. Sensory irritation: Riskassessment approaches.Regul. Toxicol. Pharmacol.2007,48(1),6-18. ( (4) N ational R esearch C ouncil. Ammonia. In Ac u te Ex p osure Guideline Levels for Selected Airborne Chemicals: Vol. 6; The NationalAcademies Press: Washington, DC, 2 0 08; p 320. ) ( (S) de la Hoz, R. E.; Schlueter, D . P.; Rom, W. N. Chronic lung disease secondary t o a mmonia in h alation in j ury: a report on th r ee c ases. Am. J. Ind. Med. 1996, 2 9 (2), 209-14. ) (6) Nagata, Y. Odor intensity and odor threshold value. Japan AirClean Assoc. 2003, 41, 17-25. (7) Sisovic, A.; Sega, K.; Kalinic, N. Indoor/outdoor relationship ofammonia concentrations in selected office buildings. Sci. TotalEnviron. 1987, 61, 73-77. ( (8) Li, Y.; Harrison , R. M. Comparison of indoor and o u tdoorconcentrations o f acid g ases, ammonia a n d th e ir associated s a lts.Environ. Technol. 1990, 11 (4), 315-326. ) ( (9) B rauer, M .; K o utrakis, P . ; Keeler, G. J.; S pengler, J . D. I ndoorand o utdoor concentrations of inorganic ac i dic aerosols and ga s es. J.Air Waste M anage. Assoc. 1991, 41 (2), 171-81. ) ( ( 10) T i dy, G.; Neil Cape, J. Ammonia concentrations in houses andpublic buildings. Atmos. Environ., Part A 1993, 27 ( 14),2235-2237. ) ( (11) Atkins, D. H. F.; L ee, D . S . Indoor concentrations of ammoniaand the potential c ontribution of humans to at m ospheric bud g ets.Atmos. E nviron., Part A 1 993, 27 (1), 1 - 7. ) ( (12) S uh, H. H . ; K o utrakis, P . ; Spengler, J . D. T he r e lationship b etween a i rborne acidity a nd a mmonia in in d oor en v ironments. J.Expo. Anal. Environ. Epidemiol. 1 994, 4 (1), 1-22. ) (13) Gomzi, M. Indoor air and respiratory health in preadolescentchildren. Atmos. Environ.1999, 33(24),4081-4086. ( ( 14) Leaderer, B. P.; N aeher, L.; Jankun, T . ; Balenger, K.; Holford, T. R .; T oth, C . ; S u llivan, J.; Wolfson, J. M.; Koutrakis, P. Indoor, ) outdoor, and regional summer and winter concentrations of PM10,PM2.5, SO4(2)-,H+, NH4+,NO3-, NH3, and nitrous acid in homeswith and without kerosene space heaters. Environ. Health Perspect.1999, 107(3),223-31. (15) Lunden, M. M.; Revzan, K. L.; Fischer, M. L.; Thatcher, T. L.;Littlejohn, D.; Hering, S. V.; Brown, N. J. The transformation ofoutdoor ammonium nitrate aerosols in the indoor environment.Atmos. Environ.2003,37(39),5633-5644. (16) Fischer, M. L.; Littlejohn, D.; Lunden, M. M.; Brown, N. J.Automated measurements of ammonia and nitric acid in indoor andoutdoor air. Environ. Sci. Technol. 2003, 37 (10), 2114-9. ( (17) L arson, T . V .; C overt, D . S . ; Frank, R . ; Ch arlson, R . J . Ammonia in the human airways: neutralization of inspired acid sulfateaerosols. Science 1977, 197 (4299), 161. ) ( (18) Sutton, M. A . ; D r agosits, U.; Tang, Y. S.; Fo wler, D . A m moniaemissions f rom non-agricultural s ources i n t he U K. Atmos. Environ.2000,34(6), 855-869. ) ( (19) Bai, Z.; Dong, Y.; Wang, Z.; Zhu, T. Emission of ammonia fr o mindoor concrete w a ll and assessment of human expo s ure. Environ. Int.2006, 32(3), 303-311. ) ( (20) Schmidt, F. M .; Vaittinen, O.; M etsala, M.; Lehto, M .;Forsblom, C.; Groop, P . H.; Halonen, L . Ammonia in b r eath a n demitted f rom skin. J. Breath Res. 2013, 7 (1), 017109. ) ( (21) N ational Science and Technology Council (U. S .); Air QualityResearch Subcommittee. Atmospheric Ammonia: Sources and Fate. A Review o f O ngoing F e deral R e search a n d Future Needs, Wa s hington, D.C., 2000. ) ( (22) Bittman, S.; Mikkelsen, R. Ammonia e m issions f r omagricultural operations: livestock. Better Crops Plant Food 2 009, 9 3 (1),28-31. ) ( (23) K lepeis, N . E.; N elson, W. C.; Ott, W. R.; Robinson, J. P.;Tsang, A . M.; Switzer, P. ; Behar,J. V.; Hern, S .C.; Engelmann, W. H.The National Human Activity Pattern Survey (NHAPS): a r e sourcefor assessing exposure to e n vironmental po l lutants. J. E x posure Sc i . E nviron. Epidemiol. 2001, 11, 2 31. ) ( (24) T uomainen, M.; Pa s anen, A.-L.; T u omainen, A.; Liesivuori,J; J uvonen, P . U s efulness of the Finnish classification of indoor climate,construction a nd finishing m aterials: comparison of i n door c l imatebetween two new blocks of flats in Finland. Atmos. Enviro n .2001 , 35(2), 305-313. ) ( (25) Jarnstrom, H .; S aarela, K .; Kalliokoski, P.; Pasanen, A. L .Reference values f o r i n door ai r pollutant concentrations in ne w ,residential buildings in Finland . Atmos. Environ. 2006, 40 (37), 7178- 7191. ) ( (26) Salonen, H . J.; P asanen, A.-L.; L appalainen, S . K.; R iuttala, H. M .; T u o mi, T. M .; Pa s anen, P. O .; Bac k , B. C .; Reijula, K. E. AirborneConcentrations o f Volatile Organic Compounds, F o rmaldehyde and Ammonia in F i nnish Office B uildings with Suspected Indoor Air Problems.J. Occup. Environ. H yg. 2009, 6 ( 3 ), 200-209. ) ( (27) Meininghaus, R. ; Ko u niali, A.; Mandin, C.; Cic o lella, A. Riskassessment of sensory irritants in indoor air-a case study in a Frenchschool. Environ. Int. 2003, 28 (7), 553-557. ) ( (28) M artin, N. A . ; F e rracci, V.; Cassidy, N.; Hoffnagle,J. A. Theapplication of a cavity ring-down s pectrometer to measurements ofambient a mmonia using traceable p r imary standard g a s m i xtures. Appl. P hys. B: L aser O pt. 2016, 122 (8),219. ) ( (29) Farmer, D . K.; Vance, M. E.; Novoselac, A.; Abbatt,J.; Abeleira,A.; Alves, M. R.; Arata, C.; Boedicker, E.; Cardoso-Saldana, F .; Corsi, R .; DeCarlo, P.; G oldstein, A. H .; G rassian, V .; Hildebrandt Ruiz, L.; J imenez, J. L.; Kahan, T. F .; Katz, E.; Nazaroff, W.; Or, V.; O’Brien,R. E.; Stevens, P.; Tian, Y.; Wang, C.; Zhou,S.; Zhou, Y. Overview of the House O bservations o f Microbial and Environmental Chemistry(HOMEChem) study. 2019, Enviro n . Sci.: Processes Impa c ts, in p r ess D OI: 1 0.1039/c9em00228f . ) (30)Novoselac, A.; Siegel, J. A. Impact of placement of portable aircleaning devices in multizone residential environments. Building andEnvironment 2009, 44(12),2348-2356. (31) Yver Kwok, C.; Laurent, O.; Guemri, A.; Philippon, C.;Wastine, B.; Rella, C. W.; Vuillemin, C.; Truong, F.; Delmotte, M.; ( Kazan, V.; D arding, M . ; Lebegue, B.; Ka i ser, C. ; Xu e ref-Remy, I.;Ramonet, M . C o m prehensive labo r atory and field test i ng of cavity ring-down spectroscopy analyzers measuring H, O , CO , CH a ndCO. Atmos. Meas. Tech. 201 5 ,8(9),3867-3892. ) ( ( 32) G o etz, J. D .; Avery, A.; Werden, B. ; Floerchinger, C. ; Fo r tner,E. C.; Wormhoudt, J . ; M assoli, P .; Herndon, S . C.; K olb, C . E .;Knighton, W. B.; P e ischl,J.; Warneke, C. ; de Go u w, J. A.; Shaw, S. L.;DeCarlo, P. F. Analysis o f local-scale background c oncentrations of m ethane and other gas-phase species in t he Marcellus S hale. Elementa- Science of the Anthropocene 2017, S, 1 -20. ) ( ( 33) K harol, S. K.; Shephard, M. W.;McLinden, C. A. ; Zhang, L.; Sioris, C. E.; O'Brien, J . M.; Vet, R.; Cady-Pereira, K. E.; Hare, E .;Siemons, J.; Krotkov, N. A. D ry Deposition o f Reactive N i trogen From Satellite Observations of Ammonia and Nitrogen Dioxide OverNorth America. Geophys. R es. Lett . 2018, 45 (2),1157-1166. ) ( (34) D uncan, S.; Tomaz, S.; Morrison G.; W ebb, M . ; Atkin, J. M.;Surratt, J. D.; Turpin, B . J. D ynamics of res i dential wa t er-solubleorganic g ases: Insights into s o urces an d sinks. En v iron. Sci. Te c hnol.2019,53(4), 1812-1821. ) ( (35) C ollins, D . B .; H ems, R. F.; Zhou, S.; W a ng, C.; Grignon, E.; Alavy, M.; S iegel, J. A.; Abbatt, J. P. D. Evidence for Gas-Surface Equilibrium C ontrol of Indoor Nitrous Acid. Environ. S c i. Technol.2018, 52(21), 12419-12427. ) ( (36) Weiss, I. M.; Muth, C.; Drumm, R.; Kirchner, H. O. K. Thermaldecomposition o f the amino aci d s glycine, cy s teine, as p artic acid,asparagine, glutamic a cid, glutamine, a rginine a nd h i stidine. B MCBiophysics 2018, 11 (1), 2. ) OI: acs.est.b American Chemical SocietyEnviron. Sci. Technol. DOI:acs.est.bnviron. Sci. Technol. 概述 氨(NH3)被美国有毒物质和疾病登记署归类为极其危险的物质,会对人体健康的造成负面影响。尽管在室外时氨的浓度通常为1-5 ppb,但在室内氨的浓度可能要高得多。室内氨的来源主要有:清洁产品的使用、烟草烟雾、建筑材料以及人类排放。氨与酸性气体以及漂白剂发生反应,会形成二次气溶胶物质,导致生态环境受到破坏、影响人类身体健康。 人们在室内的时间明显多于室外,人们大约90%的时间在家里、办公室或学校等室内建筑中度过,因此研究室内氨的浓度非常重要。由于氨具有高反应性、易溶于水、易吸附到各种表面等特点,导致很难测量出来,因此室内氨浓度的综合评价仍然是一个未被充分研究的课题。 方法 研究人员分别对冬季使用和不使用煤油加热器以及夏季使用和不使用空调的家庭中室内氨的浓度变化、办公楼内氨的浓度变化进行研究。 以前的研究通常是使用扩散取样器或带有剥离器的主动取样器来完成的,这些方法导致了许多限制,如较低的准确度和非实时测量。因此,需要提高室内测量的精度和高时间分辨率,以便能够确定影响室内动态氨浓度的因素。在此,研究人员使用了Picarro G2103分析仪对氨的浓度进行测量,使我们能够探索诸如清洁、烹饪和入住等常见室内活动以及作为供暖、通风和空调系统对室内氨的浓度变化的影响。该仪器使用光腔衰荡光谱(CRDS)分析技术来实时测量十亿分之(ppb)水平的氨。Picarro G2103分析仪放置在住宅的厨房里,位于冰箱上方,高度约2米,与炉子和烤箱的距离约为4米。仪器的入口很短,大约5厘米长,带有一个内嵌的特氟龙过滤器,以防止颗粒侵入仪器。 结果 图1总结了在以前的研究中观察到的氨浓度,表S1中给出了研究的更多细节。浓度值根据进行测量的季节和研究地点(学校、办公室或住宅)进行分类。除了一个以外,所有的数值都大于10 ppb,这个水平被认为是室外高浓度的氨。学校和办公室是氨浓度最高的地方,分别达到了440和103 ppb的最高值。家庭中的氨含量在8.1到67.7 ppb之间。 将之前研究中发现的家庭氨浓度与直方图指示的HOMEChem中发现的浓度进行比较。 直方图似乎与之前的结果一致,并提供了关于浓度值频率的额外信息。 图1.以前研究中发现的室内NH3浓度与HOMEChem实验中发现的室内NH3浓度的频率进行了比较。浓度值根据进行测量的季节(夏季或冬季)和指定的地点(学校、办公室和家庭)进行分类。 表1汇总了在上述不同活动期间测量的最小、第10个百分位数、平均值、第90个百分位数和最大NH3浓度的值。这些活动包括背景测量、夜间高温、反应时间、烹饪活动、清洁活动和入住率。背景浓度平均为31.9 ppb,每一次活动都会引起氨浓度升高。 表1.测量期间的氨浓度的变化 室内氨的浓度对房屋的室内温度有着很强的依赖性。 图2显示了两天不同的温度、相对湿度和氨浓度。图2a为空置背景的测量结果,房屋保持平均温度为25.3°C,相对湿度为57.8%,氨的平均浓度为31.9 ppb。图2B为夜间高温期间的测量结果,室内温度平均为32.7°C,相对湿度为49.1%,氨的平均浓度为65.4 ppb。夜间高温测量时的平均温度比背景测量时高出7.4°C,浓度提高了33.5 ppb。 图2.a)2018年6月15日为空置背景,将温度设置为~25.5°C;b)2018年6月24-25日为夜间高温,将温度设置为~32.2°C。 在图2中,可以很容易地观察到暖通空调系统对氨的浓度的影响:当空调打开时,冷却盘管是湿的(因为冷却盘管的温度低于空气的露点温度),温度、相对湿度和氨的浓度都会下降。当空调关闭时,温度、相对湿度和氨的浓度会立即开始上升。 图3显示了测量期间氨的浓度、室内温度和相对湿度的变化。上午10点。空调被关闭,温度从起始温度24.8°C持续上升到最终最高温度35.5°C。氨浓度也在上升,在白天期间,氨的浓度最高可达到91.6 ppb。然而在房屋冲洗期间,门窗被打开,低氨浓度的室外空气快速进入室内,室内氨的浓度迅速下降。请注意,在室内测量的氨浓度从未下降到典型的室外值,这表明室内氨的持续排放。因为在所有窗户和门打开的过程中氨的浓度都很高,这表明室内有大量的氨可以迅速释放到气相中以保持平衡。 图3. NH3响应时间活动期间的 浓度、温度和相对湿度。 黄色柱子显示了30分钟的房屋冲洗周期,即门窗打开的时间范围。 Observations and Contributions of Real-Time Indoor Ammonia Concentrations during HOMEChemLaura Ampollini, Erin F. Katz, Stephen Bourne, Yilin Tian, Atila Novoselac, Allen H. Goldstein, Gregor Lucic, Michael S. Waring, and Peter F. DeCarloEnvironmental Science & Technology 2019 53 (15), 8591-8598DOI: 10.1021/acs.est.9b02157如需更多相关信息,请联系我们
关闭-
1/8
-
2/8

还剩6页未读,是否继续阅读?
继续免费阅读全文产品配置单
爱博能(广州)科学技术有限公司为您提供《氨中室内氨的检测检测方案(氨气分析仪)》,该方案主要用于氨中室内氨的检测检测,参考标准《暂无》,《氨中室内氨的检测检测方案(氨气分析仪)》用到的仪器有null。
我要纠错
相关方案


 咨询
咨询






