方案详情文
智能文字提取功能测试中
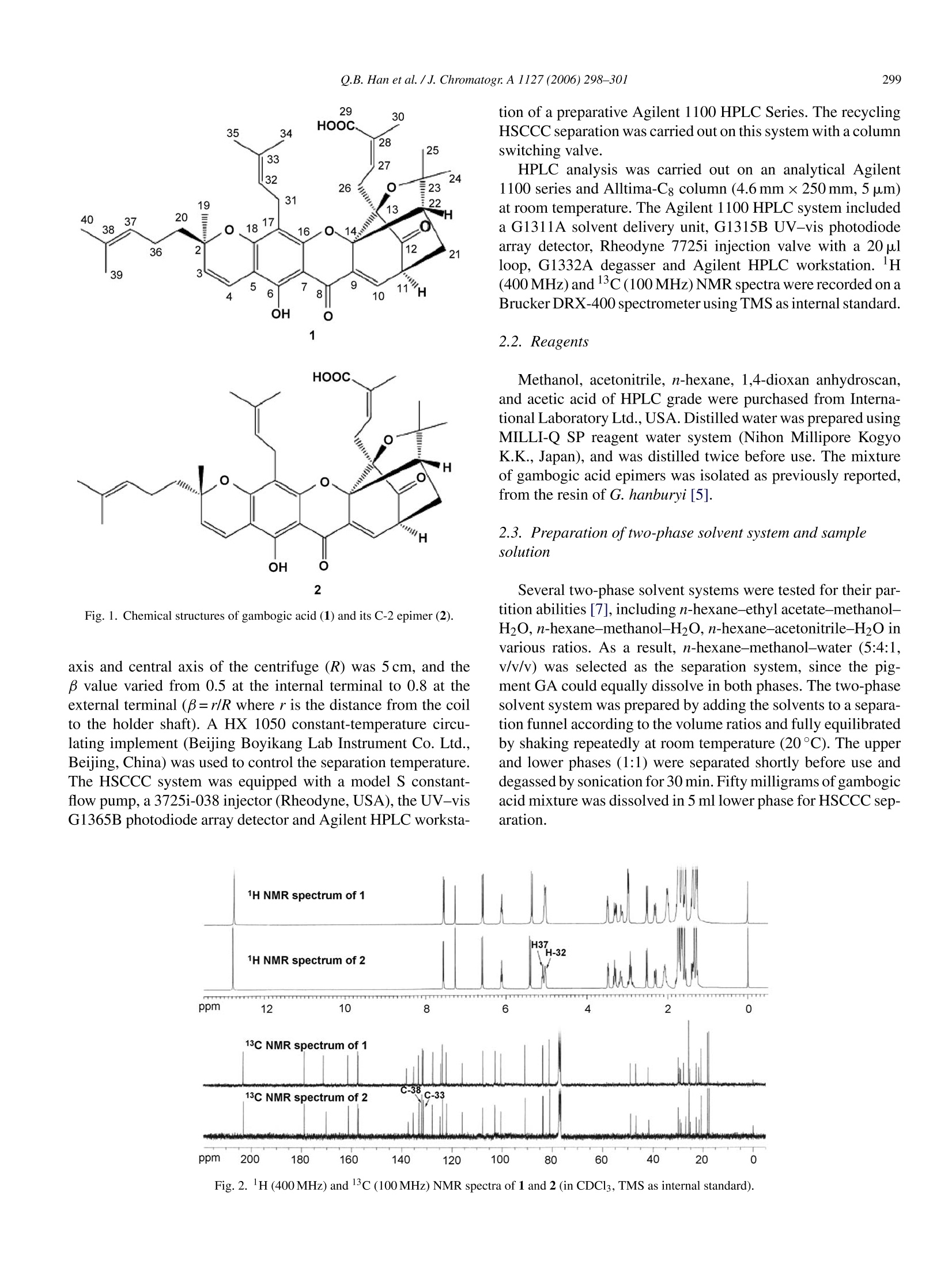
Available online at www.sciencedirect.comJOURNAL OFCHROMATOGRAPHY AJournal of Chromatography A, 1127 (2006) 298-301 299. Q.B. Han et al. /J. Chromatogr.A 1127 (2006)298-301 www.elsevier.com/locate/chroma Short communication Preparative separation of gambogic acid and its C-2 epimerusing recycling high-speed counter-current chromatography Quan Bin Han, Jing Zheng Song, Chun Feng Qiao, Lina Wong, Hong Xi Xu* Chinese Medicine Laboratory, Hong Kong Jockey Club Institute of Chinese Medicine, ShaTin N.T, Hong Kong Received 16 May 2006; received in revised form 13 July 2006; accepted 18 July 2006Available online 2 August 2006 Abstract A recycling counter-current chromatographic system was first set up with a high-speed counter-current chromatography instrument coupled witha column switching valve. This system was first successfully applied to the preparative separation of epimers, gambogic acid and epigambogicacid from Garcinia hanburyi using n-hexane-methanol-water (5:4:1, v/v/v) as the two-phase solvent system. As a result, 28.2 mg gambogic acidand 18.4 mg epigambogic acid were separated from 50 mg of mixture. Their purities were both above 97% as determined by HPLC. The chemicalstructures were then identified by their 'HNMR and 13C NMR spectra. O 2006 Elsevier B.V. All rights reserved. Keywords: Recycling counter-current chromatography; Preparative separation; Garcinia hanburyi; Epimer; Gambogic acid; Epigambogic acid 1. Introduction Gambogic acid (GA, CAS No.2752-65-0) is the principalactive component of gamboge, the resin from various Garciniaspecies including Garcinia Morella and Garcinia hanburyi.Many modern pharmaceutical studies are focused on its exten-sive and potent anti-tumor activities [1,2]. In 1970s, it had beendeveloped as an anti-tumor drug for clinical testing via intra-venous injection in China. However GA had been believed to bean inseparable C-2 epimeric mixture [3]. The stereochemistryhad not been determined until the single crystal of pyridine saltof R-epimer was obtained and then analyzed by X-ray diffrac-tion [4]. In our previous report, this epimer pair was separatedfirst by semi-preparative HPLC. The S-epimer was found morepotent against CYP 2C9 than R-epimer[5]. The chemical struc-tures of gambogic acid epimers are very similar, with only onestereochemical difference at C-2(Fig. 1). This made their lHand 13C NMR spectra extremely similar to each other (Fig.2),and also made this epimer pair difficult to separate by commonchromatography methods. Moreover, the reverse-phase HPLCseparation of these epimers with semi-preparative column was ( * Corresponding a u thor. Tel.: +852 3406 2873; f a x: +852 3 5 51 7 3 33. E-mail address: xuhongxi@hkjcicm.org (H.X. X u ). ) ( 0021-9673/$- see front matter C 2006 Elsevier B.V. A l l rights reserved.doi:10.1016/j.chroma.2006.07.04 4 ) time-consuming [5].Therefore, a simpler isolation is necessaryto achieve a better separation. High-speed counter-current chromatography (HSCCC), aspecial liquid-liquid partition chromatography without solidsupport matrix, prevents peak tailing and sample loss due toirreversible adsorption [6]. This method has been successfullyapplied in the preparative separation of natural products andchiral compounds [7-10]. In recent years, multidimensionalCCC which effectively extends the separation journey has beensuccessfully applied to natural products separation [11-13].Accordingly, it is also theoretically feasible to separate any com-pounds including epimers if the CCC separation course is longenough. This paper describes a successful separation of gam-bogic acid and its C-2 epimer using a newly built recyclingHSCCC system. 2. Experimental 2.1. Apparatus The preparative HSCCC instrument used in this study wasTBE-300A high-speed counter-current chromatography (Shen-zhen, Tauto Biotech, China) with three polytetrafluoroethy-lene preparative coils (diameter of tube, 2.6 mm, total volume,300ml). The revolution radius or the distance between the holder 2 Fig. 1. Chemical structures of gambogic acid (1) and its C-2 epimer (2) axis and central axis of the centrifuge (R) was 5 cm, and theB value varied from 0.5 at the internal terminal to 0.8 at theexternal terminal (B=r/R where r is the distance from the coilto the holder shaft). A HX 1050 constant-temperature circu-lating implement (Beijing Boyikang Lab Instrument Co. Ltd.,Beijing, China) was used to control the separation temperature.The HSCCC system was equipped with a model S constant-flow pump, a 3725i-038 injector (Rheodyne,USA), the UV-visG1365B photodiode array detector and Agilent HPLC worksta- tion of a preparative Agilent 1100 HPLC Series. The recyclingHSCCC separation was carried out on this system with a columnswitching valve. HPLC analysis was carried out on an analytical Agilent1100 series and Alltima-C; column (4.6mm x250 mm, 5 um)at room temperature. The Agilent 1100 HPLC system includeda G1311A solvent delivery unit, G1315B UV-vis photodiodearray detector, Rheodyne 7725i injection valve with a 20 plloop, G1332A degasser and Agilent HPLC workstation.H(400MHz) and C(100MHz) NMR spectra were recorded on aBrucker DRX-400 spectrometer using TMS as internal standard. 2.2. Reagents Methanol, acetonitrile, n-hexane, 1,4-dioxan anhydroscan,and acetic acid of HPLC grade were purchased from Interna-tional Laboratory Ltd., USA. Distilled water was prepared usingMILLI-Q SP reagent water system (Nihon Millipore KogycK.K., Japan), and was distilled twice before use. The mixtureof gambogic acid epimers was isolated as previously reported,from the resin of G. hanburyi [5]. 2.3. Preparation oftwo-phase solvent system and samplesolution Several two-phase solvent systems were tested for their par-tition abilities [7], including n-hexane-ethyl acetate-methanol-H2O,n-hexane-methanol-H2O,n-hexane-acetonitrile-H2O invarious ratios. As a result,n-hexane-methanol-water (5:4:1,v/v/v) was selected as the separation system, since the pig-ment GA could equally dissolve in both phases. The two-phasesolvent system was prepared by adding the solvents to a separa-tion funnel according to the volume ratios and fully equilibratedby shaking repeatedly at room temperature (20°C). The upperand lower phases (1:1) were separated shortly before use anddegassed by sonication for 30 min. Fifty milligrams of gambogicacid mixture was dissolved in 5 ml lower phase for HSCCC sep-aration. Fig. 2. 1H(400MHz) and 13c(100MHz) NMR spectra of 1 and 2 (in CDCl3, TMS as internal standard). 2.4. HSCCC separationprocedure The whole procedure was carried out in the following threesteps. Step 1: The coil column was first entirely filled withthe upper phase of the solvent system at 10 ml/min. Then theapparatus was rotated at 800rpm, while the lower phase waspumped into the column at 2.0 ml/min. After the mobile phasefront emerged and hydrodynamic equilibrium was established inthe column, 5 ml of sample solution containing 50 mg of crudeextract was injected through the injector. The separation temper-ature was controlled at 20 °C. The effluent from the outlet of thecolumn was continuously monitored at 360 nm by Agilent 1100HPLC UV-vis detector and ChemStation. Step 2: After the sam-ple injection, the switching valve was turned to form a recyclingtube. Step 3: When the target sample was completely separatedafter several CCC cycles, the switching valve was returned toits original position to release the separated samples. Each peakfraction was manually collected according to the chromatogramand evaporated under reduced pressure. The residue was dis-solved in methanol for subsequent HPLC analysis. 2.5. HPLC analysis and identification ofCCC peakfractions The epimers mixture and each HSCCC peak fraction wereanalyzed by HPLC on a Cg column eluted with CH3CN/0.1%acetic acid/1,4-dioxan (60:30:10,v/v/v). The flow rate was1.0 ml/min, and the effluents were monitored at 360 nm by a pho-todiode array detector. The HSCCC peak fraction was then iden-tified by their lH(400MHz) and 13c(100 MHz) NMR spectra. 3. Results and discussion The separation included six cycles as shown in Fig. 3. The firstcycle, about 150 min, yielded one single peak. This might sug-gest theses epimers are eluted together. Nevertheless soon afterthat, the second separation cycle exhibited a fork-like peak. Thispeak then divided into two in the subsequent cycles. At the sametime, the peak range extended from 50 min in the first cycle to150 min in the sixth cycle. This suggested that the peak wouldbe better separated in the seventh cycle. Meanwhile, the end of Fig. 3. Chromatogram of gambogic acids by preparative recycling counter-currentchromatography. Solvent system: n-hexane-methanol-water (5:4:1, v/v/v); stationary phase: upper organic phase; mobile phase: lower aqueous phase;flow-rate: 2.0 ml/min; revolution speed: 800rpm; sample: 50 mg dissolved in5ml of lower phase. Fig. 4. HPLC analyses of mixed and separated gambogic acid epimers on aC8 column eluted with CH3CN/0.1%acetic acid/1,4-dioxan (60:30:10, v/v/v).The flow rate was 1.0 ml/min, and the effluents were monitored at 360 nm by aphotodiode array detector. (A) Mixed gambogic acid epimers (reference), (B)R-epimer, (C) S-epimer. peak IIof the sixth cycle would overlap the front of the nextpeak I because the column volume was limited. The separatedpeaks were then collected as shown in Fig. 3 and analyzed byanalytical HPLC. The HPLC chromatograms of the separatedpeaks are displayed in Fig. 4. Their purities were determinedto be 97.2% and 97.5% by HPLC analysis with DAD detec-tor, respectively. Peaks I and II were identified to be gambogicacid and epigambogic acid, respectively, by HPLC comparisonwith the reference compounds and by MS, lH and 13c NMR'MS, Fspectroscopic analysis as described in our previous paper [5]. As a newly developed method, the recycling CCC showedits unique advantage in achieving effective separations throughpreventing stationary phase loss. In the case of gambogic acids,when these epimers could be separated by neither the commonHSCCC nor two coupled HSCCC, the recycling operation inone HSCCC system would be the best way to do the separation. The peak extension between separation cycles might be animpact factor for a successful separation. More separation cyclescould be carried out with a shorter peak extension. Thereforein order to shorten the peak extension, it might be useful touse a mobile phase in which the target compounds are easilydissolved. Moreover, the initial target peaks must be limited in anarrow range, which made the recycling method unsuitable forthe simultaneous separation of several compounds. The results of our studies demonstrated that recyclingcounter-current chromatography is an effective method for thepreparative separation of gambogic acid and its C-2 epimer,epigambogic acid. Acknowledgement This research is funded by the Hong Kong Jockey Club Char-ities Trust. ( [ 1] L. Zhao, Q .-L. Guo,Q.-D. You, Z . -Q. Wu, H . -Y. Gu, Biol. P harm. Bull. 27 (2004)998. ) ( [2] Z.-Q. W u, Q.-L. Guo, Q.-D. Yo u , L. Zh a o, H.-Y.Gu, Biol. Pharm. Bull. 27 ( 2004) 1769. ) ( [3] L . -J. L in, L.-Z. Lin, J.M . Pezzuto,G.A. Cordell, M agn. Reson. Chem. 31(1993)340. ) ( [4] T.J.R. Weakley, S.-X. Cai, H.-Z. Zhang, J.F.W. Keanal, J. Chem. Crystal- logr.31 (2001)501. ) ( [5]Q.-B. Han, L. Yang, Y. Li u , Y.-L. Wang, C.-F. Qiao,J.-Z. Son g , L.-J. Xu , D.-J. Yang, S.-L. Chen, H.-X. Xu, Planta Med. 72(3)(2006)281. ) ( [6] Y.Ito, Rev. Anal. Chem. 1 7 (1986) 65. ) ( [7] Y. I to, J. Chromatogr. A 1 065 (2006) 1 4 5. ) ( [8] X.-L. Cai, High-Speed Counter-Current Chromatography and Its Applica-tion, Chemical Industry Press,Beijing,2005, p. 390. ) ( [9] B. Domon, K. Hostetmann, Kovacevic, J. Chromatogr. 250(1982)149. ) ( [10] P . Franco, J. Blanc, W.R.K. O berleitner, N.M. Maier, W. Lindner, C. Min- guillon, Anal. Chem. 74 (2002)4175. ) ( [ 11 ] F.-Q. Yang, J. Quan, T.-Y. Zhang, Y. Ito, J. Chromatogr. A 803 (1998)298. ) ( [ 1 2] G .-L. Tian, T.-Y. Zhang, Y.-B. Zhang, Y. Ito , J. Chromatogr . A 945 (2002) 281. ) ( [ 1 3] Y .Wei, Y . Ito, J. Chromatogr. A111 5 (2006) 112. )
关闭-
1/4

-
2/4
还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
上海同田生物技术有限公司-高速逆流色谱仪HSCCC为您提供《藤黄酸中立体异构体分离纯化检测方案(高速逆流色谱)》,该方案主要用于化药制剂中限度检查检测,参考标准《暂无》,《藤黄酸中立体异构体分离纯化检测方案(高速逆流色谱)》用到的仪器有TBE-300A高速逆流色谱仪/制备色谱仪、TBE-200V 高速逆流色谱仪、TBE300B+AKTA高速逆流色谱仪/离心分配色谱/萃取仪/制备色谱仪、TBE-1000A制备色谱仪/萃取仪/快速分离制备色谱仪。
我要纠错
推荐专场
相关方案







 咨询
咨询

