方案详情文
智能文字提取功能测试中
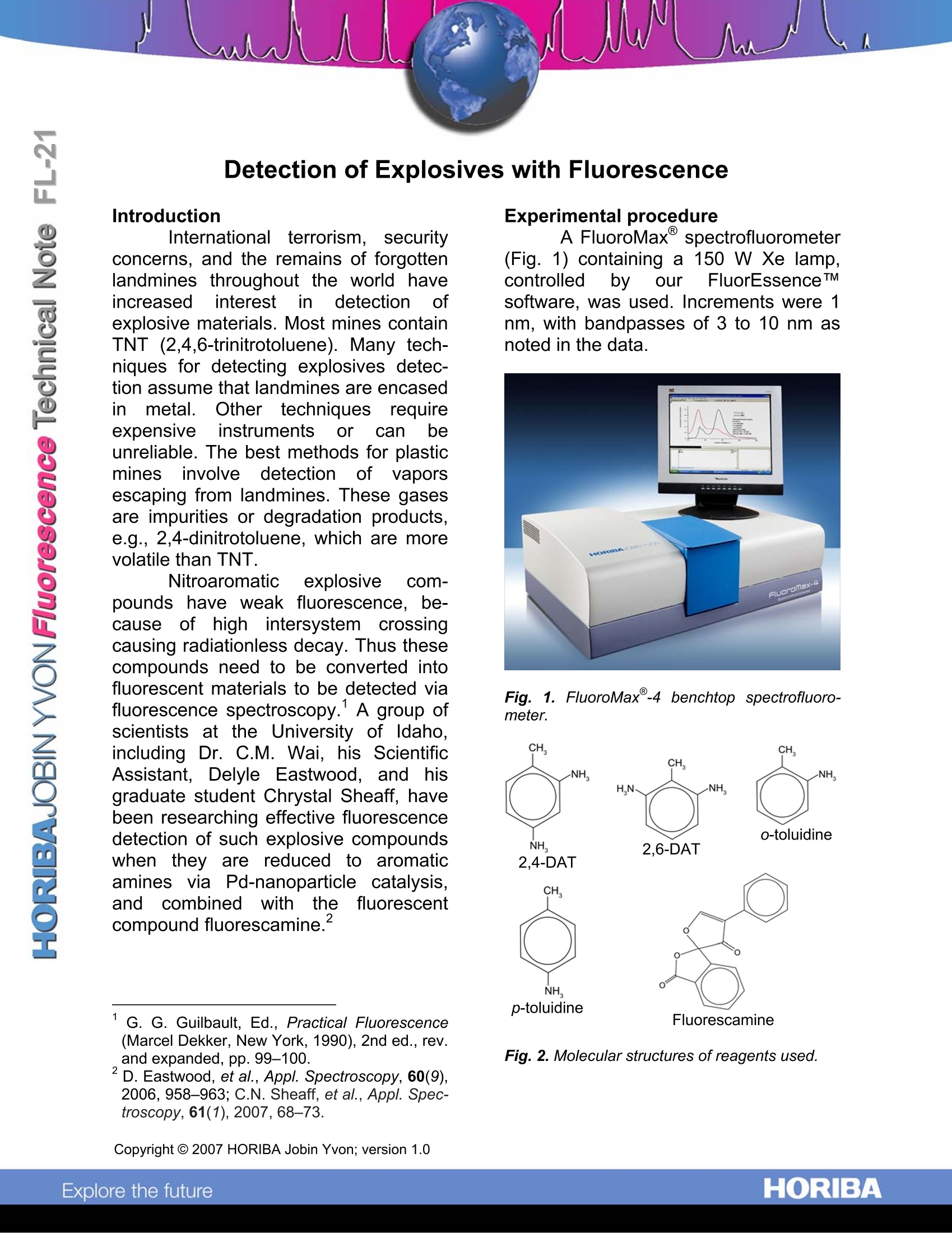
Introduction Internationalterrorism, securityconcerns, and the remains of forgottenlandmines throughout the world haveincreased interestinn detectionofexplosive materials. Most mines containTNT (2,4,6-trinitrotoluene). Many tech-niques for detecting explosives detec-tion assume that landmines are encasedinmetal.Other techniquesrequireexpensiveinstrumentsor can beunreliable. The best methods for plasticmines involve detection of vaporsescaping from landmines. These gasesare impurities or degradation products,e.g., 2,4-dinitrotoluene, which are morevolatile than TNT. Nitroaromatic explosive com-pounds have weakfluorescence., be-cause of high intersystem crossingcausing radiationless decay. Thus thesecompounds need to be converted intofluorescent materials to be detected viafluorescence spectroscopy. A group ofscientists at: tthe University of ldaho,including Dr. C.M. Wai, his ScientificAssistant, Delyle Eastwood, and hisgraduate student Chrystal Sheaff, havebeen researching effective fluorescencedetection of such explosive compoundswhen they are reduced to aromaticamines via Pd-nanoparticle catalysis,and combined with the fluorescentcompound fluorescamine.2 ( ' G. G. Guilbault, Ed., Practical F luorescence(Marcel Dekker, New York, 19 9 0), 2nd ed., rev. a nd expanded, pp.99-100. ) ( D . E a stwood, et al., Appl. S p ectroscopy, 60(9),2006, 9 58-963; C . N. Sheaff, et al., Appl. S pec-troscopy, 61(1),2007,68-73. ) Experimental procedure A FluoroMax spectrofluorometer(Fig.1) containing a 150 W Xe lamp,controlled by our FluorEssenceTMsoftware, was used. Increments were 1nm, with bandpasses of 3 to 10 nm asnoted in the data. Fig.1.1. FluoroMax-4 benchtop spectrofluoro-meter. Fig. 2. Molecular structures of reagents used. Pure ethanol was the solvent forall compounds, which themselves werethe highest purity commerciallyavailable. Compounds examined were2,4-diaminotoluene (2,4-DAT), 2,6-diaminotoluene((2,6-DAT), o-toluidine,and p-toluidine. Fluorescamine (98%)was used tocreate nitroaromaticderivatives. (See Fig. 2.) Derivatives’purities;were checked by thin-layerchromatography and H-NMR. Concen-trations down to 10-12 M were reachedin ethanol. Solutionswere stored inamber glass containerswrappedinaluminum foi to protectagainstphotodecom-position. Results and discussion An example of the FluoroMax@ssensitivity to a nitroaromatic compoundattached to fluorescamine is shown inFig. 3, with a detection limit of 10-12/2,4-DAT. Fig. 3. Excitation and emission spectra of 2,4-DAT + fluorescamine at various dilutions, blank-subtracted, and all bandpasses = 10 nm. Synchronous scans, in which theexcitation and emission monochrom-ators are simultaneously scanned, keep a constant interval (offset) between thetwo monochromators. Synchronousspectra were measured at a 9 nm offsetwith bandpasses = 3 nm and integrationtime =1 s. A comparison of excitation,emission, and synchronousscanssisisshown in Fig. 4. The top plot shows howsimilar all the emission scans are forfour differentcompounds. Nc : themuchnarrowerpeakforthesyn-chronous scan of 2,4-DAT comparedwith its excitation and emission scans. Fig. 4. (Top) Emission spectra of 4×10M 2,6-DAT, 4×10M 2,4-DAT, 4×10M o-toluidine,and 4×10’M p-toluidine. (Bottom) Comparisonof excitation,synchronous, and emission spectraof 4 ×105M 2,4-DAT. All spectra are blank-subtracted in EtOH, with 3 nm bandpass. Similar emission, excitation, andsynchronousspectra weretaken ofseveral decomposition products of TNT.Results are shown in Table 1. NoticehowEeventthe peaks scanned withsynchronousmonochromators.whilenarrower and distinguishable, are stillvery similar in wavelength. Compound Excitation Emission Synchronous peak, peak, peak, FWHM FWHM FWHM (nm) (nm) (nm) 2.6-DAT 287,27 335,51 309,10 o-toluidine 282,32 340,49 314,16 2,4-DAT 290,39 350,50 319,14 p-toluidine 285,42 349.50 325,15 Table 1. Peak values with full-widths at half-maximum fitrom emission., excitation. andsynchronous scans of TNT-decompositionproducts. Therefore.,to further enhancesensitivity and selectivity tospecificcompounds in(explosives, secondderivatives were applied to the spectra.Amongttheinmathematicalfunctionsincludewdith1 our exclusive Origin-based FluorEssence TMsoftware areSavitzky-Golay smoothing and deriv-ative calculations by averaging slopesbetweentwo adjacent data points.Professor Wai and coworkers found thatfive-pointsmoothing gave lthe bestresolution with second derivatives. An example of a four-componentmixture of 2,6-DAT,o-toluidine,2,4-DAT, and p-toluidine in ethanol withconcentrations in the 105 M range isshown in Fig.5. (These were found tohave detection limits via synchronousscanning of 2.6×10-6M, 2.3×10-6 M,6.7 10’M, and1..77 10-M.respectively.))W hWihlilee the synchronousspectrum (upper plot) has only a singlevisiblepeak,thesecond derivative (lower curve) reveals the presence ofeach component clearly. Fig.5. (Top) Synchronous spectrum of a mixtureof 2,6-DAT(4x10-5M), o-toluidine (1x105M),2,4-DAT (2 x10M), and p-toluidine (1 x 10M)in EtOH; bandpasses=33nm, blank-subtracted, integration time = 1 s. (Bottom) Sec-ond derivative of the top graph, with componentsidentified at 309, 314, 319, and 325 nm. Conclusions Professor Wai's research is thefirst to show that synchronous scanningplus the second derivative can distin-guish among four likely reduction prod-ucts in a TNT-based explosive mixture.Synchronous scanning itself has advan- tages over other identification methods,because it is rapid, sensitive, and selec-tive.Taking the second derivative helps,in addition, to distinguish various prod-ucts whose spectra overlap. The use ofan agent (fluorescamine, in this case) toform a derivative compound reaches alower limit of detection. HORIBA JobinYvon spectrofluorometers are capableof recording and analyzing weak fluo-rescence such as that presented here. Acknowledgements This work was partially supportedby a grant from the Department of De-fense-Air Force Office of Scientific Re-search (AFOSR, F49620-03-0361) Origin is a registered trademark of OriginLabCorporation. ( USA: H ORIBA Jobin Yvon In c ., 3 8 80 Park Avenue, Edison, NJ 08 8 20-3012, Toll-Free:+1 - 866-jobinyvon Tel: +1-732-494-8660, F ax: + 1 - 732-549-5125, E - mail: info@jobinyvon.com,www.jobinyvon.com France: H ORIBA Jobin Yvon S . A . S., 1 6-18, rue du Canal , 9116 5 Longjumeau Cedex, ) ( T el: +33 (0) 16454 13 00,F a x: + 33 (0) 1 69 09 93 1 9 , w ww.jobinyvon.f r Japan: H ORIBA Ltd., J Y Optical Sales Dept, Hig a s h i-Kanda, Daij i Building, 1-7-8 Higashi-Kanda ) ( Chiyoda-ku, T okyo 1 0 1-0031, T e l:+81 (0)3 3861 8231, w w w.jyhoriba.jp Germany: +49(0)89 462317-0 I taly: +39 0 2 57603050 U K: +44 (0) 20 8204 8142 ) Copyright C HORIBA Jobin Yvon; version .HORIBAExplore the future HORIBAExplore the future Professor Wai’s research is the first to show that synchronous scanning plus the second derivative can distinguish among four likely reduction products in a TNT-based explosive mixture. Synchronous scanning itself has advantages over other identification methods, because it is rapid, sensitive, and selective. Taking the second derivative helps, in addition, to distinguish various products whose spectra overlap. The use of an agent (fluorescamine, in this case) to form a derivative compound reaches a lower limit of detection. HORIBA Jobin Yvon spectrofluorometers are capable of recording and analyzing weak fluorescence such as that presented here.
关闭-
1/4
-
2/4
还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
HORIBA(中国)为您提供《芳香族硝基化合物炸药中2,4DAT检测方案(分子荧光光谱)》,该方案主要用于其他中含量分析检测,参考标准《暂无》,《芳香族硝基化合物炸药中2,4DAT检测方案(分子荧光光谱)》用到的仪器有HORIBA高灵敏一体式FluoroMax-4荧光光谱仪。
我要纠错
推荐专场
相关方案




 咨询
咨询





