方案详情文
智能文字提取功能测试中
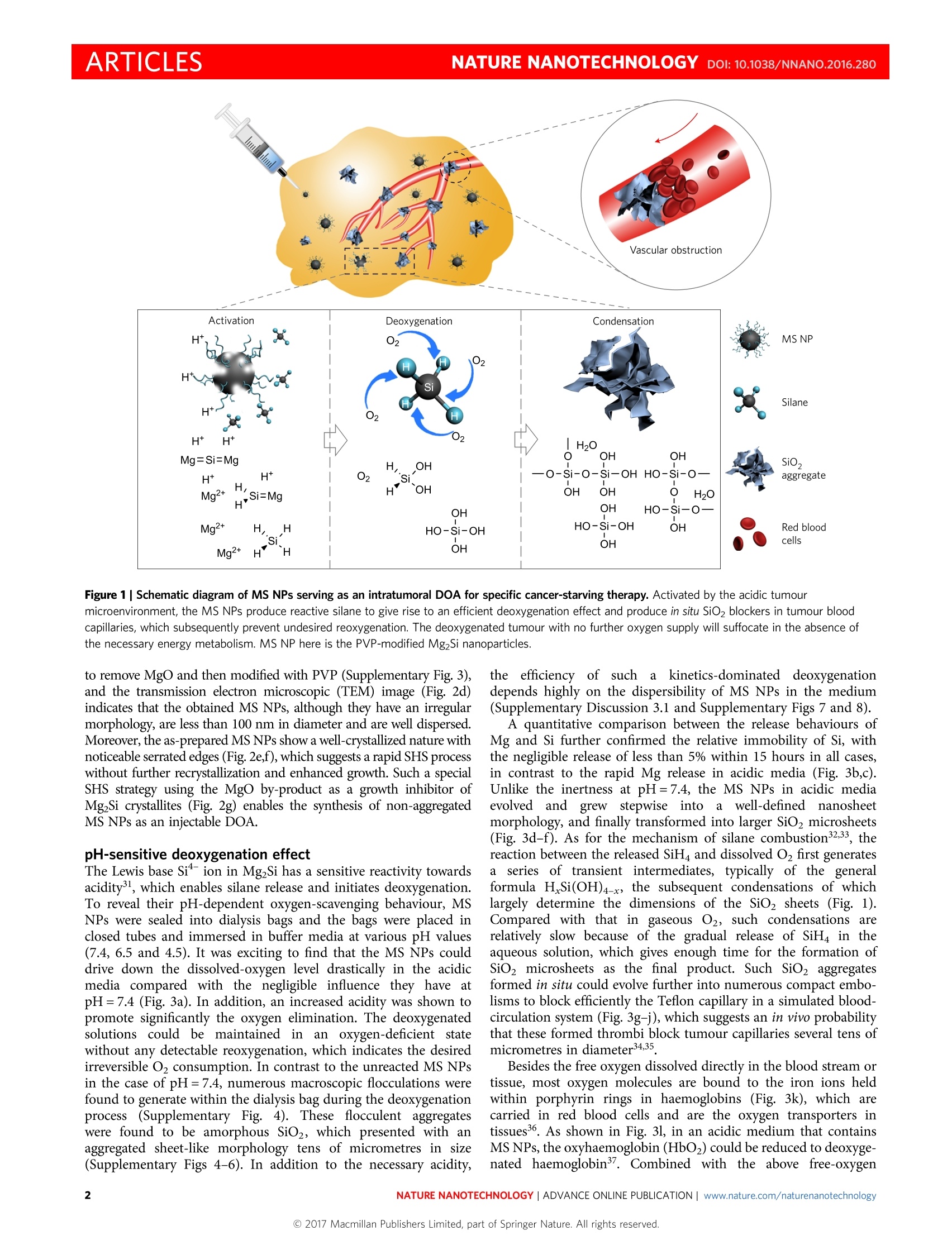
ARTICLESPUBLISHED ONLINE: 9 JANUARY 2017 |DOI: 10.1038/NNANO.2016.280naturehanotechnology ARTICLESNATURE NANOTECHNOLOGYDOI:10.1038/NNANO.2016.280 Magnesium silicide nanoparticles as adeoxygenation agent for cancer starvation therapy Chen Zhang', Dalong Nil, Yanyan Liul, Heliang Yaol, Wenbo Bu12* and Jianlin Shil* A material that rapidly absorbs molecular oxygen (known as an oxygen scavenger or deoxygenation agent (DOA)) hasvarious industrial applications, such as in food preservation, anticorrosion of metal and coal deoxidation. Given thatoxygen is vital to cancer growth, to starve tumours through the consumption of intratumoral oxygen is a potentially usefulstrategy in fighting cancer. Here we show that an injectable polymer-modified magnesium silicide (Mg2Si) nanoparticlecan act as a DOA by scavenging oxygen in tumours and form by-products that block tumour capillaries from beingreoxygenated. The nanoparticles are prepared by a self-propagating high-temperature synthesis strategy. In the acidictumour microenvironment, the Mg2Si releases silane, which efficiently reacts with both tissue-dissolved and haemoglobin-bound oxygen to form silicon oxide (SiO2) aggregates. This in situ formation of SiO2 blocks the tumour blood capillariesand prevents tumours from receiving new supplies of oxygen and nutrients. anotechnology has been making significant improvements inNcancer therapy through various functional nanomaterials thathave emerged, including drug-delivery nanocarriers-,nanoradiosensitizers-7, photothermal nanoagents8-10 and photody-namic nanoplatforms11-13. However, there is a paucity of researchon materials for tumour starvation, a well-known and promisingtherapeutic strategy. With cancer starvation, rapid tumour growthcan beinhibited by stopping the blood1 supply that deliversoxygen and nutrients to the tumour14. Feasible cancer-starving pro-tocols and drugs with clinical prospects are therefore highly sought. Anti-angiogenic therapy is a cancer-starvation method that inhi-bits unwanted blood-vessel growth in tumours and blocks thesupply of nutrients and oxygen15-17. Given that oxygen is vital forcancer growth and the lack of it can lead to hypoxia-induced celldeath18-20, we hypothesize that tumours could also be starved bythe direct removal of intratumoral oxygen. Although various deox-idants are available, such as iron powders, ascorbic acid and sul-fites21-23, it remains a challenge to design a drug that fulfils thevarious requirements of a cancer-starving agent. Such an agentmust be biocompatible, which therefore excludes any toxic speciessuch as heavy metals. Furthermore, it must have a high deoxygena-tion efficiency and the characteristics of a long-term oxygen scaven-ger with tumour-tissue specificity. Moreover, for practical purposes,it should be easily injectable by a syringe. Another intractableproblem in cancer-starvation therapy is the rapid reoxygenation ofas-deoxygenated tumours through undamaged blood vessels. Here we report polyvinyl pyrrolidone (PVP)-modified Mg2Sinanoparticles (MS NPs) as a qualified deoxygenating agent(DOA) to realize specific tumour-starving therapy (Fig. 1). Inresponse to the mildly acidic tumour microenvironment thatresults from the unique glycolytic metabolism², the MS NPs areexpected to be activated specifically to scavenge environmentaloxygen via the following reactions: Interestingly, the intermediate silane (SiH4) is notorious as a self-igniting gas in air that is an explosive hazard2. Such a highly reactivenature with O, is utilized subtly in this study, based on the findingthat the dissolved SiH4 slowly released from MgzSi (equation (1))could serve as a safe oxygen scavenger (equation (2)). In theory,one mole of Mg2Si can consume two moles of oxygen molecules(equation (3)), which suggests a high oxygen-consumption effi-ciency. Biocompatible Mg,H2O and SiO2 are the only deoxygenat-ing products, which reduces any concerns about the potential sideeffects. Very importantly, fortunately the SiO2 aggregates generatedin situ could block tumour capillaries and maintain intratumoralhypoxia by choking off the undesirable blood supplies (Fig. 1). Synthesis and characterizations of MS NPs Microscale Mg2Si particles conventionally prepared from a solid reac-tion in an inert atmosphere are too large to acquire any dispersibility inliquid media (Supplementary Figs 1 and 2)26.27. To obtain injectableMS NPs with tissue penetration, we developed a special self-propagat-ing high-temperature synthesis (SHS) approach in an Oz/Ar mixed gasatmosphere. In this approach,O2 could exothermally react with excess Mg,which induces the subsequent combination reaction between Mg andSi. The scanning electron microscopic (SEM) image in Fig. 2a revealsthat the product before washing appears to contain numerous micro-particles; an X-ray diffraction pattern analysis (Supplementary Fig. 1)showed this to be a mixture of MgzSi and MgO. From the magnifiedSEM image and the corresponding energy-dispersive spectroscopy(EDS) element mapping of a cracked particle (Fig. 2b,c), these micro-particles were confirmed to be of a defined core-shell morphology,a MgzSi/MgO core within a thin outer MgO shell. From the well-known grain boundary pinning effect28-30, as a high-melting-pointphase that surrounds the Mg Si particles, the MgO by-product couldinhibit their unwanted grain growth, and the outer MgO shell prevents the inner MgzSi from oxidation. The MgzSi nanoparticles were washed State Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai200050, China.Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East ChinaNormal University, Shanghai 200062, China. *e-mail: jlshi@mail.sic.ac.cn; wbbu@chem.ecnu.edu.cn Figure 1| Schematic diagram of MS NPs serving as an intratumoral DOA for specific cancer-starving therapy. Activated by the acidic tumourmicroenvironment, the MS NPs produce reactive silane to give rise to an efficient deoxygenation effect and produce in situ SiO2 blockers in tumour bloodcapillaries, which subsequently prevent undesired reoxygenation. The deoxygenated tumour with no further oxygen supply will suffocate in the absence ofthe necessary energy metabolism. MS NP here is the PVP-modified Mg2Si nanoparticles. to remove MgO and then modified with PVP (Supplementary Fig. 3),and the transmission electron microscopic (TEM) image (Fig. 2d)indicates that the obtained MS NPs, although they have an irregularmorphology, are less than 100 nm in diameter and are well dispersed.Moreover, the as-prepared MS NPs show a well-crystallized nature withnoticeable serrated edges (Fig.2e,f), which suggests a rapid SHS processwithout further recrystallization and enhanced growth. Such a specialSHS strategy using the Mgo by-product as a growth inhibitor ofMg2Si crystallites (Fig. 2g) enables the synthesis of non-aggregatedMS NPs as an injectable DOA. pH-sensitive deoxygenation effect The Lewis base Si ion in MgSi has a sensitive reactivity towardsacidity3, which enables silane release and initiates deoxygenation.To reveal their pH-dependent oxygen-scavenging behaviour, MSNPs were sealed into dialysis bags and the bags were placed inclosed tubes and immersed in buffer media at various pH values(7.4, 6.5 and 4.5). It was exciting to find that the MS NPs coulddrive down the dissolved-oxygen level drastically in the acidicmedia compared with the negligible influence they have atpH=7.4(Fig. 3a). In addition, an increased acidity was shown topromote significantly the oxygen elimination. The deoxygenatedsolutions could be maintained inan oxygen-deficient statewithout any detectable reoxygenation, which indicates the desiredirreversible O2 consumption. In contrast to the unreacted MS NPsin the case of pH=7.4, numerous macroscopic flocculations werefound to generate within the dialysis bag during the deoxygenationprocess (Supplementary Fig. 4). These flocculent aggregateswere found to be amorphous SiO2, which presented with anaggregated sheet-like morphology tens of micrometres in size(Supplementary Figs 4-6). In addition to the necessary acidity, the efficiency of suchaa kinetics-dominated deoxygenationdepends highly on the dispersibility of MS NPs in the medium(Supplementary Discussion 3.1 and Supplementary Figs 7 and 8). A quantitative comparison between the release behaviours ofMg and Si further confirmed the relative immobility of Si, withthe negligible release of less than 5% within 15 hours in all cases,in contrast to the rapid Mg release in acidic media (Fig. 3b,c).Unlike the inertness at pH=7.4, the MS NPs in acidic mediaevolveadn da ngdrew stepwise intooaa well-defined nanosheetmorphology, and finally transformed into larger SiO2 microsheets(Fig. 3d-f). As for the mechanism of silane combustion32.33, thereaction between the released SiH4 and dissolved O first generatesa series of transient intermediates, typically of the generalformula H.Si(OH)4-x, the subsequent condensations of whichlargely determine the dimensions of the SiO sheets (Fig.1).Compared with that in gaseous O2, such condensations arerelatively slow because of the gradual release of SiH4 in theaqueous solution, which gives enough time for the formation ofSiO2 microsheets as the final product. Such SiO2 aggregatesformed in situ could evolve further into numerous compact embo-lisms to block efficiently the Teflon capillary in a simulated blood-circulation system (Fig. 3g-j),which suggests an in vivo probabilitythat these formed thrombi block tumour capillaries several tens ofmicrometres in diameter34,35 Besides the free oxygen dissolved directly in the blood stream ortissue, most oxygen molecules are bound to the iron ions heldwithin porphyrin rings in haemoglobins (Fig. 3k), which arecarried in red blood cells and are the oxygen transporters intissues36. As shown in Fig. 3l, in an acidic medium that containsMS NPs, the oxyhaemoglobin (HbO2) could be reduced to deoxyge-nated haemoglobin. Combined with the above free-oxygen a b g Mg,Si-PVP(MSNPs) Figure 2| Synthesis of MS NPs and their morphological and microstructural characterizations. a, SEM image of the product derived from the solid reactionof Mg and Si mixtures in an Ar/O2 atmosphere. Scale bar, 10 um. b,c, The magnified SEM image (b) and corresponding EDS element mapping images (c) ofa cracked microparticle in a reveal its well-defined core-shell structure. Scale bars, 1 um. d, TEM image of highly dispersed MS NPs. Scale bar, 500 nm.e, TEM image of a representative MS NP. Scale bar, 20 nm. The inset is the corresponding selected-area electron-diffraction pattern. Scale bar,5 nm.f, Close-up high-resolution TEM (HRTEM) image showing the serrated edge of the nanoparticle (highlighted by black arrows). Scale bar, 5 nm. Each SEMand TEM experiment was performed twice on two parallel samples, and representative images are presented. g, Schematic diagram of the SHS approach toprepare injectable MS NPs. A powder mixture of Si and excess Mg was heated in an Ar/O2 atmosphere, in which O2 could react exothermally with excessMg to induce the combination reaction between Mg and Si that forms the core-shell microparticles with cores of an Mg2Si/MgO mixture inside thin outerMgO shells. The MgO by-product that surrounds the Mg2Si nanoparticles could inhibit their unwanted growth. The dispersible PVP-modified Mg2Sinanoparticles can be obtained after MgO removal and PVP modification. elimination, such an acidity-specific deoxygenation effect of MSNPs on bound oxygen suggests the desirable complete oxygenscavenging in the tumour. Furthermore, the silane intermediatewas revealed to be a moderate and safe reducer that underwent anirreversible eight-electron-reduction process in aqueous solutions(Supplementary Discussion 3.2 and Supplementary Fig.9), whichenables the additional scavenging of hydroxyl radicals and hydrogenperoxide (Supplementary Fig. 10), common but potentially harmfulspecies in cancer38,39. In vitro deoxygenation for cancer-cell starvation The MS NPs and their deoxygenating products were shown to be ofnegligible cytotoxicity in normal incubations(SupplementaryFig. 11). To evaluate their pH-sensitive deoxygenation in vitro, anacidic culture medium of pH=6.5 was employed to simulate the mod-erately acidic extracellular environment in tumours, and the MCF-7human breast adenocarcinoma cell was chosen because its superficiallocation enabled a practical tumour-starving administration. Excitingly,a fast acidity-specific deoxygenation effect was observed in theculture media with the addition of MS NPs (Supplementary Fig. 12),which suggests an efficient intracellular-oxygen scavenging inan acidic environment, as evidenced by the significantly inten-sified red fluorescence of the intracellular O2 level indicator[Ru(dpp)3]Cl2 (dpp, 4,7-diphenyl-1,10-phenanthroline)(Fig. 4a,b)40.Furthermore, by incubating with 8F-labelled fluoromisonidazole(F-MISO), a commercial hypoxia molecular probe capable ofselectively binding to hypoxic cells, the subsequent 18F positronemission tomography (PET)/computed tomography (CT) imaginggave a quantitative assessment of the cell hypoxia level (Fig. 4c,d). Compared with the cases without acid or MS NP addition, theF-MISO radioactive signals in the PET image are much strongerfor a combined acid and MS NP addition. This and the increasedlevels of hypoxia-inducible factor la (HIF-1a) protein expres-sion (Fig. 4e,f) evidently confirm the indispensability of the con-current presences of MS NPs and acidity in creating severeintracellular hypoxia. In this study, there were no detectable influences on the cell growthinduced by the MS NPs or acidity alone (Supplementary Fig. 13).However, their synergism-induced intracellular hypoxia turnedout to be an effective inhibitor of cell proliferation. Such a generatedhypoxia could result in a much-suppressed cell growth under theadministration of higher concentrations of MS NPs, in contrastwith the exponential growth profile in the control group (Fig. 4g).With a low concentration (2 mM) of MS NPs, the oxygen depletionof the culture medium (Fig. 4h) induces a relatively low level of cellhypoxia (Fig. 4d), but still gives rise to a cell-proliferation inhibitionrate of about 50% in 24 hours. An increased dose of 5 mM couldefficiently improve the inhibition effects, however, the further increasein dose of 10 mM gave no significant increments in oxygen-consump-tion rate, intracellular hypoxia level and suppression efficiency on cellgrowth (Fig. 4b,d,g and Supplementary Fig. 12), which indicates ahypoxic saturation at about 5 mM. When kept in the deoxygenatedenvironment, these hypoxic cells experienced a dramatic change inthe morphology that featured a successive cellular divorce, membranedamage and membranolysis (Supplementary Fig. 14). Moreover, incontrast to that in normoxic cells, the mitochondria in the deoxyge-nated cells were damaged irreversibly, as characterized by serious swel-ling, membrane breakage and plasma spillover, in succession (Fig. 4i). Figure 3|pH-sensitive deoxygenation effect of the as-synthesized MS NPs. a-c, Change in the dissolved-oxygen level over time (a) and correspondingreleasing behaviours of Mg (b) and Si (c) of the MS NP-containing media of different pH values at 37°C. The experiment was performed in triplicate andindependently (n=3), data are mean ±s.d. d-f, Representative SEM images of the evolution of nanoscale MS NPs (d) during the deoxygenation process inthe medium of pH=6.5 show a stepwise transformation in morphology and size (solids collected at 4 h (e) and at 12 h (f)) into SiO2 microsheets.Scale bars, 1 um. Each SEM experiment was performed twice on the samples collected from two independent deoxygenation processes. g, Digital photographof the equipment for simulating the circulation of the MS NP-dispersed solution of pH=6.5 in a Teflon capillary (highlighted by the yellow dashed line).The red arrow indicates the anticlockwise circulation. Scale bar, 5 cm. h, Digital photographs of the initial free-flowing (taken at 2 min) Teflon capillary andthe later blocked one after the reaction. Scale bars, 1 mm. i, The SEM image of the blocked capillary (as shown in the left photograph) section shows acompact embolism within it. The yellow arrows indicate the interface between the capillary and the embolism. Scale bar, 100 um.j, Magnified SEM image(top left) of the region in the red square in i and the corresponding element mappings confirm the embolism of SiO2. Scale bar, 10 um. Each SEMexperiment and corresponding element mapping was performed three times on three independent blocked capillary sections. k, This schematic structure ofhaemoglobin shows the oxygen molecule bound to the central ferrous ion. The deoxygenating efficiency depends on the reducing capacity of silane to thehaemoglobin-bound oxygen molecules.l, UV-vis absorption spectra of the blood buffer solution after different treatments (control, pH=7.4 buffer; MS NPs,pH=7.4 buffer + MS NPs; H*, pH=6.5 buffer; MS NPs+H*, pH=6.5 buffer +MS NPs). The experiment was performed three times, and representativeUV-vis absorption spectra are presented. The two characteristic absorption peaks centred at 542 and 577 nm are assigned to HbO2, and the singlebroadened band around 555 nm is assigned to deoxygenated haemoglobin (Hb). The MS NPs show a reducing behaviour on HbO2 only in an acidic medium, which confirms their acidity-specific deoxygenating feature in scavenging the haemoglobin-bound oxygen molecules. a.u., arbitrary units. These suffocated mitochondria are believed to have lost their respir-atory and metabolic ATP production functions, which finally resultsin cellular necrosis and/or apoptosis41. To confirm further the exclusive role of hypoxia in cell killingand exclude any potential influences of the formed SiO2 micro-sheets, a special experimental set-up was designed. Cells were Figure 4|In vitro assessments of the MS NP-mediated deoxygenation for cell starvation. a, Representative confocal images of the intracellular O2 level indicator [Ru(dpp)3]Cl2-stained MCF-7 cells after treatments in different culture media of pH= 7.4 or 6.5 and co-incubated with various concentrations ofMS NPs (0, 2, 5 and 10 mM) for 2 h. Scale bars, 50 um. The experiment was performed twice on two independent culture disks for each group, andrepresentative images are presented. The increased red fluorescence indicates a decrease in the intracellular oxygen level. b, The correspondingdissolved-oxygen concentrations in the culture media. Five independent culture disks per group were used for the dissolved-oxygen-level measurements(n=5), ***p<0.001, Student's two-sided t-test. c,d, Representative PET images (c) of 18F-labelled MISO binding cells in different groups as shown in a andcorresponding quantitative 18F radioactivity levels (d) normalized to that of the control. All the images in c fall within the same radioactivity scale as shownon the left. The cell hypoxia level is proportional to the normalized 18F radioactivity. The experiment was performed three times and independently, andrepresentative images (c) and quantitative results (d, n=3, mean±s.d.) are presented. e,f, HIF-1o protein expressions (e) of MCF-7 by the differenttreatments described in a and the corresponding quantitative levels (f) normalized to actin. g,h, MCF-7 cell proliferation rates (g) and corresponding changesin dissolved-oxygen level (h) in the acidized culture media that contain different concentrations of MS NPs. The experiment was performed in sextuplicate in96-well microplates (n=6 per group), mean±s.d.,*P<0.05, **P<0.01 and ***P<0.001, Student's two-sided t-test. i, Bio-TEM images of the MCF-7 cellsco-incubated with MS NPs for various time intervals show the substantial damage to intracellaur mitochondria in the MS NP-induced hypoxic condition. Themitochondria are highlighted by white (control), red (1h) and yellow (4h) dashed ovals. Each bio-TEM experiment was performed on three cell sectionsobtained from independent culture cases, and the representative images are presented. In contrast to the normal mitochondria in normoxic MCF-7 cells witha well-defined length-diameter aspect ratio (control),the hypoxia-induced irreversible mitochondria destruction features serious swelling, membranebreakage and plasma spillover of the mitochondria. Scale bars, 2 um. j, Flow cytometry apoptosis assay based on AnnexinV-FITC and PI strained MCF-7 cellscultured in the medium of pH=7.4 and incubated with various concentrations of MS NP solutions (pH=6.5) in the non-contact mode. The experiment wasperformed three times, and representative results in one test are present. The enhanced apoptosis positively associated with the concentration of MS NPsconfirms the exclusive role of hypoxia in cancer-cell killing, and excludes any potential influences of the formed SiO2 microsheets. incubated in a medium of pH =7.4 in a covered container, but withan open microcentrifuge tube that contained a MS NP solution ofpH=6.5 being placed inside the covered container(Supplementary Fig. 15). The tube within would undergo a fastdeoxygenation, which would lead to gradually elevated hypoxialevels in the outer culture medium. Despite the slow intracellularoxygen consumption, the cell activity was similarly drivendown, accompanied by a significantly increased cell apoptosis andmitochondria damage (Fig. 4j and Supplementary Fig. 16), whichexcludes possible causes of cytotoxicity other than hypoxia.More importantly, MS NP-mediated deoxygenation is generally applicabletto other cell lines for proliferation inhibition(Supplementary Fig. 17), and for intracellular hydroxyl radical orhydrogen peroxide elimination (Supplementary Fig. 18). All theseresults imply that MS NPs are a favourable acidity-specific DOAin cell proliferation inhibition. In vivo cancer-starving therapy The potential qualification of MS NPs to serve as an in vivo DOA fortumour-starving therapy was then evaluated on bilateral 4T1 xeno-grafted tumour-bearing mice, with MS NPs intratumorally injectedinto the right tumours and saline into the left ones as a control. Figure 5|MS NP-mediated tumour-starving therapy in vivo. a,b, Time-course PA sO2 mapping and high-resolution US images of the subcutaneous 4T1xenografted tumours in a BALB/c mouse that received an i.t. injection of saline (a) or MS NP solution (b). Scale bars, 2 mm. c, Quantitative comparison ofthe time-course intratumoral sO2 levels after different treatments. The PA/US imaging experiment was performed three times on three independent 4T1tumour-bearing mice per group, and representative images (a and b) and quantitative results (c, n=3,mean±s.d.) are presented. d, Time-course normalizedintratumoral dissolved-oxygen levels measured at various distances (1, 3 and 5 mm) from the tumour surface after the i.t. injection of MS NPs indicate slightdifferences in the intratumoral O2-consumption dynamics at different locations. The experiment was performed on three independent 4T1 tumour-bearingmice (n=3,mean±s.d.). e, Representative time-dependent depth profiles of the intratumoral dissolved-oxygen level in the MS NP-mediated deoxygenationprocess show a fast decrease in the oxygen tension of the whole tumour. The experiment was performed four times on independent 4T1 tumour-bearingmice, and the result of a representative one is presented. f, Representative 18F-MISO PET/CT images before (early PET/CT) and after (late PET/CT)different-treatment administrations show a significantly enhanced hypoxia after MS NP injection. Top row, transverse-view PET/CT images. Bottom row,coronal view PET images. The CT reconstruction image (middle) shows the bilateral 4T1 xenografted tumours (indicated by arrows) in vivo, which were i.t.injected with saline (left) and MS NP solution(right) after the early PET/CT imaging. Scale bar, 1 cm. All the images fall within the same radioactivity scaleshown on the right. g, Quantitative analysis of the ratio between the late and early SUV of 18F-MISO in the individual tumour confirms the much-enhancedand well-maintained hypoxia after MS NP administration, in contrast to the negligible changes in the intratumoral hypoxia level with saline treatment. The invivo PET/CT imaging experiment was performed on three independent bilateral 4T1 tumour-bearing mice, and representative images (f) and the quantitativeresults (g, n=3, mean±s.d.) are presented. h, Time-dependent relative tumour growth curves after different treatments. The experiment was performed onfive bilateral 4T1 tumour-bearing mice (n=5 for both the control and MS NP groups), mean ±s.d., ***p<0.001, Student's two-sided t-test. The control groupwith saline injection exhibited about an eightfold increase in the relative tumour volume in 16 days, but the MS NP-starved tumours showed a much-suppressed growth. ij, HIF-1o and p53 protein expressions of the tumours received different treatments (i) and their corresponding quantitative levels (j)normalized (norm) to Actin. k, Representative H&E-stained sections of 4T1 xenografted tumour tissue collected at 1, 6 and 24 h after i.t. injection of the MS NPs. Scale bars, 50 um. n=4 sections per group, and each section was obtained from different tumours. Through the co-registration of multiwavelength photoacoustic (PA)and B-Mode ultrasonic (US) images, the change of intratumoralblood oxygen saturation (sO2), that is, the haemoglobin-boundoxygen level, was monitored quantitatively in real time. The sO2value of the control group remained almost unchanged at about37%, equal to that pre-injection (Fig. 5a,c), which confirms theslight hypoxic nature of a solid 4T1 tumour in this period and thenegligible influence of saline itself on the intratumoral sOz.Significantly, there was a drastic sO2 reduction from ten minutespost-injection of the MS NPs, and such a reduction trend continuedfor up to three hours with the intratumoral blood oxygen beingalmost completely scavenged (Fig. 5b,c), which indicates the effi-cient deoxygenation of HbO2. Simultaneously, the dissolved freeoxygen in the tumour tissue was shown by a gold-standard oxygen microelectrode (Supplementary Fig. 19) to be equally andthoroughly eliminated (Fig. 5d,e). The marked fluctuation in theoriginal microprofile of the intratumoral dissolved oxygen indicatesa heterogeneous oxygen distribution in a solid tumour, and suchheterogeneity was eliminated drastically down to a long-lastingzero-oxygen tension after the MS NP injection. In theory, a favour-able microenvironment for cell growth relies essentially on thebalance between the haemoglobin-bound oxygen and intratissuedissolved oxygen, so the complete depletion of both these in thetumour will inevitably lead to severe hypoxia and subsequent cellu-lar necrosis and apoptosis. Furthermore, in vivo 18F-MISO PET/CT imaging was performedto assess the intratumoral hypoxia level and potential mis-hypoxiain non-tumorous regions. Consistent with the PA/US results, the 0.5h 24h Si/Mg Pre 5 min 30 min Figure 6| Evolutions of MS NPs during the cancer-starving therapy in vivo. a, Time-course of normalized concentrations of Mg and Si in 4T1 xenograftedtumours after the i.t. injection of MS NPs shows a much slower elimination of Si than that of Mg. The measurements were performed on three independent4T1 tumours (n=3), mean±s.d. b, Microdistributions of ratiometric Si/Mg (mol) in tumour tissue at different time points (0.5, 2 and 24 h) after i.t. injectionof MS NPs scanned by LD-IMS. The experiment was performed twice on two tumours with collection at each time point, and representative images arepresented. Large numbers of regions with a higher Si/Mg ratio were detected in prolonged post-injection durations, which indicates a fast Mg leaching butpersistent Si retention. c-g, Representative time-course TEM images reveal the evolutions of MS NPs in the morphology and crystallization during thedeoxygenation in vivo. Top images in c-g are the overview TEM images of solid intratumoral extractant collected at different time intervals after MS NPinjection. Scale bars, 500 nm. The HRTEM image (bottom left) and corresponding Fourier transformation (bottom right) in c confirm the well-crystallized MSNPs before deoxygenation. Scale bar, 5 nm. The dashed lines in the bottom HRTEM image of d highlight the encapsulated Mg2Si nanocrystals in theamorphous matrix at 0.5 h after the in vivo deoxygenation. Scale bar, 5 nm. The magnified TEM images (bottom left) and corresponding electron diffractionpatterns (bottom right) in e and f show the amorphous products that evolved from the MS NP-injected tumours after 2 and 12 h in the tumour. Scale bars,100 nm. Arrows in g mark the broken regions in a SiO2 microsheet collected 60 h after MS NP administration, and confirms their gradual degradationbehaviour in the late stage. h,i, Corresponding K-edge core-loss EEL spectra (h) and quantitative analysis of O, Mg and Si in c-g, indicate significant oxygencapture and magnesium elimination, accompanied by the drastic valence reversal of silicon from -4 to +4. The TEM and EELS experiments were performedthree times on the solid collections extracted from three independent 4T1 tumours, and representative TEM images (c-g), EELS (h) and quantitative results(i, n=3, mean±s.d.) are presented. j, 2Si MAS NMR spectra of 4T1 tumours collected at 0.5, 12 and 60 h post MS NP administration confirm the incompletecondensation of the SiO2 microsheets formed in situ in the tumours. k, Representative coronal-view T1-weighted MRI images of 4T1 tumours at 4 h post i.t.injection of saline (left) or MS NPs (right). Images were obtained in routine pre-5 and 30 min post-Magnevist-enhanced scans. I, Quantitative T1 signalintensities of complete tumours in the corresponding MRI images show a much slower enhancement of the DOA-treated tumours. The MRI experiment wasperformed on three independent bilateral 4T1 tumour-bearing mice, and representative images (k) and quantitative results (l, n=3, mean±s.d.) arepresented. The slight gradient signal enhancement in the right tumour in 30 min is attributed to the tissue-penetration effect of the small-molecular Gd complex of Magnevist, as it is an impossible delivery pathway of red blood cells. early PET/CT images showed equivalent hypoxia levels before treat-ment, which confirmed the slightly hypoxic nature of the bilateraltumours at this stage (Fig. 5f). Despite the unavoidable nucleardecay and metabolic loss of the 18F-MISO radioactivity in vivo, a much-enhanced 18F-MISO uptake was observed in the PET/CTimages one hour after MS NP administration (Fig.5f,g). More excit-ingly, in the PET/CT images at three hours, the hypoxic region wasfound to cover the whole tumour region without any detectable newly created hypoxia region in normal tissues around the tumour.As a negative control, after the subcutaneous injection of MS NPs,the blood sOz and oxygen tension in the normal tissue werefound to have no significant variation (Supplementary Fig. 20),which further confirms the acidic sensitivity and specificity of MSNPs in vivo, and thus eliminates concern as to the potential sideeffects on non-cancerous tissue of normal pH value. Excitingly, the DOA-starved tumours showed a much slowergrowth than the control group with saline injection only, which con-firms the intratumoral (i.t.) administration of MS NPs to be a highlyefficient inhibitor of tumour growth with no detectable toxicity inthe main organ tissues (Fig. 5h and Supplementary Figs 21 and 22).Importantly, intratumoral hydrogen peroxide was consumed atthe same time (Supplementary Fig. 23). Moreover, in accordancewith the extensively reported hypoxia-induced p53 accumulation,a significant HIF-1a upregulation through such an exogenousdeoxygenation was also found to rejuvenate the p53 apoptoticpathway in the tumour (Fig. 5i,j), which in turn can lead to therapid apoptosis of cancer cells43. As expected, in addition to thedirect hypoxia-induced mitochondria damage demonstrated in vivo(Supplementary Fig. 24), pathological haematoxylin and eosin(H&E) staining assays also showed that after one hour the tumoursstarved by the DOA feature obvious and significant fibrosis, necrosisand apoptosis, and such damage became substantially more seriousover 24 hours (Fig. 5k). Evolution of MS NPs during deoxygenation in vivo To understand further this DOA-induced cancer-starving therapy,the time-course evolutions of the intratumoral MS NPs were theninvestigated. In line with the in vitro findings, most of the Si wasdetained and gradually degraded in the tumour, in contrast to thefast elimination of Mg (Fig. 6a). A ratiometric Si/Mg map scannedby laser desorption/ionization mass spectrometry (LD-IMS) gavethe relative microdistributions of Si and Mg in the tumour(Fig. 6b). At 0.5 hours post-partial injection of the MS NPs, theobserved uniform distribution of Si/Mg at an atomic ratio ofaround 0.8 confirms their quick penetration even under the highintratumoral interstitial fluid pressure44. Afterwards, chromatic-contrast Si-rich microaggregates emerged and further grew intolarger ones, accompanied by an overall increased ratio of Si to Mg(Supplementary Fig. 25). Owing to the highly heterogeneousnature of tumour acidity4, the regions with a higher Si-to-Mgratio are regarded as the ones of stronger acidity. Fortunately, thisnon-uniform acidity distribution did not result in an undesireddeoxygenating heterogeneity, as evidenced from the notable whole-tumour oxygen scavenging and hypoxia generation (Fig. 5b,d-f). From a more microscopic view, the time-course TEM images andelectron-energy-loss spectra (EELS) reveal a stepwise transform-ation of intratumoral MgzSi crystalline nanoparticles into amor-phous microsheets. Such a transformation is accompanied by asignificant oxygen capture and magnesium elimination, and, moreevidently, by the drastic valence reversal of silicon from -4 to +4(Fig. 6c-i). From the 2Si solid-state magic angle spinning nuclearmagnetic resonance (MAS NMR) results (Fig. 6j), the initialdeoxygenating product was found to be unsaturated H Si(OSi)4-xspecies, namely the amorphous substances that encapsulate thecrystalline MS NPs (Fig. 6d). These intermediates were furtheroxidized and condensed into SiO2 microsheets rich in silanolgroups, as suggested by the well-defined Q2 and Qs species. Thisfinding evidently confirms a mild and stepwise dehydration-condensation mechanism of the intratissue SiOz-microsheet for-mation, which enables these dynamically generated aggregates toact as an efficient tumour capillary blocker to stop the circulationof red blood cells (Supplementary Fig. 26). Furthermore, fast-scanning magnetic resonance imaging (MRI)on the bilateral 4T1 tumour-bearing mice by using Magnevist as the T1-contrast agent was employed to estimate the permeabilityof intratumoral vasculature. The saline-injected left tumourquickly brightened within five minutes, in contrast to a muchslower signal enhancement in the MS NP-injected right tumour(Fig. 6k,l), which confirms the much-diminished tumour vasoper-meability caused by the SiO microsheets formed in situ. Such alargely inhibited vasopermeability contributes to the long-termintratumoral hypoxia by blocking the further supply of bloodoxygen (Supplementary Fig. 27). Additionally, the thrombogenicphenomenon was found absent with the injection of exogenousSiO2 microsheets (Supplementary Fig. 28), with no significanttumour-growth inhibition observed (Supplementary Figs 29 and 30)because of their much-less-probable penetration into the capillaries. More interestingly, these slow-condensed SiO2 microsheets werefound to degrade gradually into small molecular silicic acids thatfeature the recognizable Qi and Q2 species in the late stage(Fig. 6j) and account for their complete elimination in thetumour in about seven days (Fig. 6a and Supplementary Fig. 25).Fortunately, after this the tumour still maintained the severehypoxia (Supplementary Fig. 27), which may be ascribed to thehypoxia-induced damage of tumour vasculature, essentially theapoptosis/necrosis of the intratumoral vascular endothelial cells. In summary, we show a proof-of-concept study that PVP-modified Mg Si nanoparticles can be used as a DOA to starvetumours. These nanoparticles efficiently consume intratumoraloxygen in the acidic tumour microenvironment. Furthermore, theamorphous SiO2 microaggregate by-products can block tumourblood capillaries and prevent reoxygenation before undergoingdegradation. In the context of a clinical translation, the adminis-tration of an intravenous (i.v.) injection was evaluated. Despite thebiocompatibility of the nanoparticles (Supplementary Figs 31-36),this injection route resulted in only a slight enhancement intumour hypoxia (Supplementary Fig. 37) and, consequently, aless-effective tumour inhibition (Supplementary Fig. 38). Ourresults show that, in addition to traditional anti-angiogenesistherapy, intratumoral deoxygenation using nano-DOA offers apromising alternative cancer-starvation therapy. Further studieson surface modification to achieve longer vascular circulation(Supplementary Fig. 39) will be required for a targeted tumouraccumulation with the nanoparticles. Received 15 November 2015; accepted 17 November 2016;published online 9 January 2017 References 1. Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat.Nanotech. 2, 751-760 (2007). 2..Ganta, S., Devalapally, H., Shahiwala, A. & Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 126,187-204(2008). 3.Petros, R. A.& DeSimone, J. M. Strategies in the design of nanoparticles fortherapeutic applications. Nat. Rev. Drug Discov. 9, 615-627 (2010). 4.Mura, S., Nicolas,J. & Couvreur, P. Stimuli-responsive nanocarriers for drugdelivery. Nat. Mater. 12, 991-1003 (2013). 5.Chithrani, D. B. et al. Gold nanoparticles as radiation sensitizers in cancertherapy. Radiat. Res. 173, 719-728 (2010). 6.Zhang, C. et al. Marriage of scintillator and semiconductor for synchronousradiotherapy and deep photodynamic therapy with diminished oxygendependence. Angew. Chem. Int.Ed. 127,1790-1794 (2015). 7.Xing, H. Y. et al. Computed tomography imaging-guided radiotherapy bytargeting upconversion nanocubes with significant imaging andradiosensitization enhancements. Sci. Rep. 3, 1751 (2013). 8.Cheng, L., Wang, C., Feng, L. Z., Yang, K. & Liu, Z. Functional nanomaterials forphototherapies of cancer. Chem. Rev. 114, 10869-10939 (2014). 9.Huang,X. H., Jain,P. K., El-Sayed, I. H.& El-Sayed, M. A. Plasmonic photothermaltherapy (PPTT) using gold nanoparticles. Laser Med. Sci. 23, 217-228 (2008). 10. Lal, S., Clare, S. E. & Halas, N. J. Nanoshell-enabled photothermal cancertherapy: impending clinical impact. Acc. Chem. Res. 41, 1842-1851 (2008). ( 1 1 . Ferrari, M. Cancer nanotechnology: opportunities and challenges. Nat. Rev.Cancer 5, 161-171 ( 2005). ) ( 12. Idris, N. M. et al. In vivo photodynamic therapy using upconversion nano p articles a s r emote-controlled nanotransducers. Nat. Med. 1 8,1 5 80-1585(20 1 2). ) ( 1 3 . Ge, J . C. et a l. A graphene quantum do t photodynamic therapy agent with high s inglet oxygen g eneration. Nat. Commun. 5 , 4596 (2014). ) ( 14. Folkman, J., Merler, E, Abernathy, C. & Williams, G. Isolation of a tumor factor r esponsible f or angiogenesis. J . Exp. Med. 133,275-288 (1971). ) ( 15. Jain, R. K. Normalizing tumor vasculature with anti-angiogenic therapy: a newparadigm for combination t h erapy. Nat. Med. 7, 987-989(2 0 01). ) ( 16. Marx, J. A b oost f or t umor s t arvation. Science 301, 452-454(20 0 3). ) ( 17. Kerbel, R. S . & Kamen,B . A. The a n ti-angiogenic b a sis of metronomicchemotherapy. Nat. R ev. Cancer 4, 423-436 (2004). ) ( 18. Shimizu, S. et al . Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature 374, 811-813 (1995). ) ( 19. Semenza,G. L . Life with o xygen. Science 3 18, 6 2-64 (2007). ) ( 20. Pedraza, E . , Coronel, M. M. , F r aker, C. A., Ricordi, C. & Sta b ler, C. L. Preventinghypoxia-induced cel l death in beta cell s and islets via hydrolytically activated, oxygen-generating b iomaterials. Proc. Natl Acad. Sci. USA 1 09, 4245-4250(2012). ) ( 21. Lindskog, P. & Arbstedt, P. Iron powder manufacturing t e chniques: a brief r eview. Powder Metall. 2 9, 14-19 (1986). ) 22. Smith, J. P., Ramaswamy, H. S. & Simpson, B. K. Developments in foodpackaging technology. Part II.:Storage aspects. Trends Food Sci. Tech.1,111-118(1990). ( 23. Vermeiren, L., D evlieghere, F., Van Beest, M., De K ruijf, N. & D ebevere, J. Developments in the active pa c kaging of foods. Tre n ds Food Sci. Tech . 10, 7 7- 8 6(1999). ) ( 24. Gerweck, L. E . & Seetharaman, K. Cellular pH gradient in tumor versus no r maltissue: potential exploitation for the treatment of ca n cer. Cancer Res. 56,1194-1198 (1996). ) ( 25. Britton, L . G. Combustion hazards of silane and its chlorides. Process Saf. Prog. 9, 16-38(1990). ) ( 26. Zhang, L. M ., Leng, Y. G., Jiang, H . Y., Chen, L. D. & Hirai, T . Synthesis of Mg2Sii _ Ge, thermoelectric compound by solid phase reaction. M a ter. Sci. Eng. B 86,195-199 (2001). ) ( 27. Liang, J . W. e t al. Nanoporous silicon prepared through air-oxidation demagnesiation of Mg2Si an d properties of its lithium ion batteries. C hem. Commun. 51,7230-7233 (2015). ) ( 28. Rios, P. R. Overview no. 62: a theory for grain boundary pinning by particles.Acta Metall. 35 , 2805-281 4 (1987). ) ( 29. Kim, J . H., Dou, S. X., Shi, D. Q. , Rindfleisch, M . & Tomsic, M. Study of MgOformation and structural defects in in s i tu processed MgB2/Fe wires. Sup e rcond. Sci. Tech. 20, 1026(2007). ) 30. Andrievski, R. Nanocrystalline high melting point compound-based materialsJ. Mater. Sci. 29, 614-631(1994). ( 31. Nandi, K., Mukherjee, D., Biswas, A. & Acharya, H . Optimization of ac i dconcentration, temperature and par t icle size of magnesium silicide, obtainedfrom r i ce husk, for the production of silanes. J. M ater. Sci. Lett. 12, 1 248-1250(1993). ) ( 32. F ukutani, S.,Uodome, Y., Kunioshi, N. & J inno, H. Combustion reactions in sila n e- a ir flames I. Flat premixed f lames. B ull. Chem. Soc. J pn 64, 2328-2334 (1 9 91). ) 33. Miller, T.,Wooldridge, M. & Bozzelli, J. Computational modeling of the SiH+O2reaction and silane combustion.Combust. Flame 137,73-92(2004). . 34. Jain, R. K. Transport of molecules in the tumor interstitium: a review. CancerRes. 47, 3039-3051(1987). 35. Liotta, L. A., Kleinerman, J. & Saidel, G. M. Quantitative relationships ofintravascular tumor cells, tumor vessels, and pulmonary metastases followingtumor implantation. Cancer Res. 34,997-1004 (1974). 36. Alayash, A. I. Oxygen therapeutics: can we tame haemoglobin? Nat. Rev. DrugDiscov. 3, 152-159 (2004). 37. Hui, Y.Y. et al. Wide-field imaging and flow cytometric analysis of cancercells in blood by fluorescent nanodiamond labeling and time gating. Sci. Rep.4,5574 (2014). 38. Hussain, S. P., Hofseth, L. J. & Harris, C. C. Radical causes of cancer.Nat. Rev.Cancer 3, 276-285(2003). ( 39. Lopez-Lázaro, M. Dual role of hydrogen peroxide in ca n cer: possible relevance t o c ancer chemoprevention and therapy. C a ncer Lett. 252, 1-8 (2007). ) ( 40. P apkovsky, D. B. & Dm i triev, R. I. Bi o logical de t ection by optical oxygen s ensing. Chem. S o c. Rev. 42, 8700-8732 (2 0 13). ) ( 41. Solaini, G., Baracca, A., Lenaz, G. & Sgarbi, G. Hypoxia and mitochondrial o xidative metabolism. B BA Bioenergetics 1797, 1 1 71-1177 (2 0 10). ) 42. Sermeus, A. & Michiels, C. Reciprocal influence of the p53 and the hypoxicpathways. Cell Death Dis.2, e164 (2011). ( 43. Graeber, T. G. et al. Hypoxia-mediated selection o f cells with diminished a poptotic potential in s olid tumours. N ature 379, 8 8 -91 (1996). ) 44. Heldin, C. H., Rubin, K., Pietras, K. & Ostman, A. High interstitial fluidpressure-an obstacle in cancer therapy. Nat. Rev. Cancer 4,806-813 (2004). 45. Helmlinger, G., Yuan, F, Dellian, M. & Jain, R. K. Interstitial pH and pO2gradients in solid tumors in vivo: high-resolution measurements reveal a lack ofcorrelation. Nat. Med. 3,177-182 (1997). Acknowledgements This work was financially supported by the National Natural Science Foundation of China (Grant no. 51372260 and no. 51132009) and the Shanghai Excellent Academic Leaders Program (Grant no.16XD1404000). We thank C. Zuo and C. Cheng from the Department of Nuclear Medicine, Changhai Hospital, for providing the 18F-MISO PET/CT imaging; J. Qu from GE Healthcare, Shanghai, for technical assistance with the MRI and P. Lu, Q. Li, L. Zhang and J. Feng from the Shanghai Institute of Ceramics, Chinese Academy of Sciences, for useful discussions. Author contributions C.Z., W.B. and J.S. conceived the experiments and were responsible for most of the data collection. D.N. and Y.L. helped with the biomedical evaluations. H.Y. contributed to the TEM measurement and structure analysis. C.Z., W.B. and J.S. analysed the experimental data and wrote the paper. All the authors discussed the results and commented on the manuscript. Additional information Competing financial interests The authors declare no competing financial interests. . Methods Materials and reagents. Magnesium powder (200 mesh,99%), silicon powder(200 mesh, 99.9%), ammonium hydroxide (NH.H2O) and PVP (average relativemolecular mass of 30,000 (PVP30)) were purchased from Sinopharm ChemicalReagent Co. All the chemical agents were of analytical grade and used directly withno further purification. Ultrapure water used throughout the experiments wasprepared using an ELGA water purification system (PURELAB Classic). Synthesis of MS NPs. The MS NPs were synthesized via a SHS approach. Typically,100 mmol of magnesium powder and 40 mmol of silicon powder were mixed andplaced in a25 ml alumina crucible. The mixture was then heated at 500°C under anAr/O2 (5%O2) atmosphere for 3 h with a ramping rate of 10°℃ min.After anatural cooling to room temperature, the resultant product was immersed in 200 mlof 95% ethanol solution that contained 2 g of PVP30, and then treated byultrasonication at 60℃ for 5 h to hydrate MgO adequately. The subsequentsuspension was gently centrifuged at 5,000 revolutions per minute (r.p.m.) for10 min to eliminate the insoluble Mg(OH)2 and other large particles. The dispersedMS NPs were then collected by centrifugation at 13,000 r.p.m. for 15 min, andwashed with ethanol three times. The control Mg2Si particles were preparedaccording a traditional solid method through heating a mixture of the powdersthat contained 80 mmol magnesium and 40 mmol silicon at 600℃ under a pureAr atmosphere for 3 h with a ramping rate of 10℃ min. Material characterization. X-ray diffraction was measured on a Rigaku D/MAX-2250 V at Cu Ka (入=0.154056 nm) with a scanning rate of 5° minin the 20 rangeof 20-70°. SEM images and the corresponding element distribution were obtainedwith an FEI Magellan 400 microscope. TEM images coupled to EELS were acquiredon a JEM-2100F microscope equipped with a Gatan GIF 963 filter operated at200 kV. Element concentration was determined by inductively coupled plasmaoptical emission spectrometry (ICP-OES) using an Agilent 700 Series instrument.The size distribution was measured by dynamic light scattering using a Nano-ZS90Malvern Zetesizer. Fourier transform infrared spectroscopy (FTIR) spectra weremeasured on a Nicolet 7000-C spectrometer using KBr pellets. pH-dependent deoxygenization in vitro. Aliquots of 0.5 ml of the as-preparedMS NP solution (0.1 M MS NPs, benchmarked against the Mg concentrationdetermined by ICP-OES) were sealed into dialysis bags with a cutoff molecularweight of 3,000 Da. Then, these dialysis bags were put into the plastic tubes andimmersed into 30 ml of buffer media at various pH values (7.4, 6.5 and 5.4), whichwere all pre-treated at 37℃ to reach an equilibrium of dissolved oxygen (~6 ug mlat 37℃). The subsequent pH-dependent deoxygenization assessments were carriedout in a shaker at 37℃ with a shaking speed of 100 r.p.m. At given intervals, theconcentration of the dissolved oxygen was measured by a Unisense oxygenmicroelectrode, and 1 ml of the medium was collected to determine the releasingconcentration (ug ml) of Mg and Si ions by using ICP-OES with 1 ml of freshbuffer medium returned. The time-dependent releasing ratios of Mg (Tn(Mg)) and Siion (rn(Si)) were, respectively, calculated as in equations (4) and(5): 30cn(Mg) , Ci(Mg) Tn(Mg) -×100% (4) minitial(Mg) where cn(Mg) and cn(Si) are the measured concentrations of Mg and Si in the nthcollection, and the constants minitial(Mg) and minitial(Si) are the total amount oforiginal Mg (4,860 ug) and Si (2,800 ug), respectively. The time-dependent dissolvedO2 consumption (no,) and Mg release (nMg) were calculated using equation (6): where cn(o,) is the measured concentration (ug ml) of dissolved O in the nthmeasurement. All the experiments were carried out in triplicate and independently.Data were taken as mean±s.d., and the best-fitting line was based on the pseudofirst-order kinetics.At the end, the observed white flocculations in the dialysis bag inthe cases of pH=6.5 and 5.4 were collected, washed with water and freeze-dried forFTIR analysis Acidity-sensitive deoxygenation of the bound O2 in haemoglobin. At 20 min afterthe addition of MS NPs (0.1M, 20 ul) to 5 ml of the buffer media at pH=7.4 and pH=6.5, which contained 10 ul of fresh blood collected from a female BALB/cmouse (age of 7 weeks) and 5 mM ETDA disodium salt as anticoagulant, theiT.absorptions ranged from 450 to 650 nm of the solution (control, pH=7.4 buffer;MS NPs, pH=7.4 buffer + MS NPs;H*, pH=6.5 buffer; MS NPs+H,pH=6.5 buffer + MS NPs) were recorded by an ultraviolet-visible (UV-vis)spectrophotometer (Shimadzu UV-3600). The experiment was performed threetimes, and representative UV-vis absorption spectra were used. Typically, HbO2 ischaracterized by the two absorption peaks at around 542 and 577 nm, and thedeoxygenated haemoglobin by a single band at around 555 nm (ref. 46). Cell culture. All the cell lines used were obtained from the Cell Bank of ShanghaiInstitute of Biochemistry and Cell Biology, Chinese Academy of Sciences, where theywere characterized systematically by DNA fingerprinting, isozyme detection andmycoplasma detection. All these cell lines were well expanded, cryopreservated infrozen vial in liquid nitrogen, and recovered every three months for use from the thesame batch of frozen vial. The NRK-52E rat renal proximal tubular cell line, MCF-7human breast adenocarcinoma cell line and Hela human cervical cancer cell linewere cultured in high-glucose DMEM (Gibco) and the BRL-3A rat liver cell line and4T1 mouse breast tumour cell line in RPMI 1640 medium (Gibco). The culturescontained streptomycin (100 mg ml-), penicillin (100 units ml) and 10% heat-inactivated fetal bovine serum at 37 ℃ in a humidified atmosphere with 5% CO2. All thecell lines were not tested further for mycoplasma contamination in the culture process Assessment of the cellular hypoxia by an optical probe. MCF-7 cells were firstseeded (1×10 cells in 1 ml DMEM of pH=7.4) in the CLSM-exclusive culture diskand allowed to adhere overnight at 37 ℃ under 5% CO2 gas. The cells were thenincubated with [Ru(dpp)3]Clz (Sigma-Aldrich) at a concentration of 10 ug mlfor12 h, washed with PBS three times to remove the free [Ru(dpp)3]Cl2. After theaddition of 1 ml of fresh DMEM culture media to the different treatments (mediumof pH=7.4, medium of pH=7.4 containing 10 mM MS NPs and medium ofpH=6.5 containing 0, 2, 5 and 10 mM MS NPs), the MCF-7 cells were furtherhermetically cultured in a confocal dish for 2 h at 37C. The concentration changesin the dissolved oxygen of the culture media was measured by using a Unisenseoxygen microelectrode. The level of intracellular O2 was evaluated by detecting thefluorescence of [Ru(dpp)3]*(ex=450 nm, 入em =610 nm (ex, excitation; em,emission) with confocal laser scanning microscopy (FV 1000, Olympus). Five andtwo independent culture disks per group were carried out for the dissolved-oxygen-level measurements and the fluorescent imaging, respectively. F-MISO PET/CT assessment on the MS NP-induced hypoxia in vitro. Theinitial [o]-misonidazole was purchased from Huayi Science and Technology(Jiangsu). The subsequent [F]-MISO was produced on a cyclotron PETtrace (GE),in which the [o]-misonidazole in a 1.5 ml silver target was irradiated with protonsto realize the l8o(p,n) F nuclear reaction. MCF-7 cells were first seeded (1×10cells in 10 ml DMEM of pH=7.4) in the culture disk and allowed to adhereovernight at 37 ℃under 5%CO2 gas. After being hermetically co-incubated with 10ml of fresh DMEM culture media with different treatments (medium of pH=7.4,medium of pH=7.4 that contained 10 mM MS NPs and medium of pH=6.5 thatcontained 0, 2, 5 and 10 mM MS NPs) at 37C for a further 2 h, the cells wereimmediately washed, trypsinized, harvested and resuspended in 1 ml of PBS in anEppendorf microcentrifuge tube (1.5 ml) with the addition of 10 pCi of 8F-MISO in10 ul of saline. After further co-incubation for 15 min, the PET/CT imaging wasperformed after the cells were sufficiently washed with PBS and precipitated bycentrifugation at 2,000 r.p.m. for 5 min. In vitro cancer-cell starvation. MCF-7 cells were seeded (1×10 cells in 100 ul ofDMEM per well) in sextuplicate in 96-well microplates, and allowed to adhereovernight. The acidized culture medium of pH=6.5 was obtained through theaddition of hydrochloric acid to a concentration of about 12 mM (ref. 47), whichshowed a negligible influence on the cell growth. The media were then replaced byfresh media of pH = 6.5 that contained 0, 2, 5 and 10 mM MS NPs. At given intervalsafter hermetical incubation, the dissolved O2 level was measured by a Unisenseoxygen microelectrode, and the time-dependent cell viabilities (v) were tested by a. typical MTT method (MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). The cell growth rate (g (%)) was calculated using equation (8): where vo is the corresponding cell viability measured immediately without furtherincubation.Similar procedures were performed on HeLa and 4T1 cell lines toestimate the generalization of the MS NP-mediated deoxygenation for cellstarvation. The DOA-starved MCF-7 cells collected at given times (1 and 4 h) werefixed with 2.5% glutaraldehyde and 1% osmium acid, dehydrated, embedded andthen sliced into ultrathin sections for Bio-TEM (JEM-1400+) observation of theintracellular mitochondria. Non-contact deoxygenation in vitro. To simulate the non-contact deoxygenation,an open microcentrifuge tube that contained MS NPs was co-incubated with the cells inside the culture disk (Supplementary Fig. 15a). Briefly, MCF-7 cells wereseeded (1×10 cells in 2 ml DMEM of pH=7.4) in the culture disks and allowed toadhere overnight. Then, the microcentrifuge tubes that contained 1 ml of MS NPbuffer solution (pH= 6.5) at concentrations of 0,2, 5 and 10 mM MS NPs wereco-incubated with the cells inside the culture disk. The dissolved-oxygen levels of thesolution inside the microcentrifuge tube and culture medium outside were measuredby a Unisense oxygen microelectrode (n=3 for each time point). These originallysealed disks are not recoverable after the measurement. After a 24 h hermeticalincubation, the cells were immediately trypsinized, harvested, rinsed andresuspended in 500 ul of binding buffer that contained 2 uM Annexin V-FITC and4.5 uM propidium iodide (PI (Annexin V-FITC/PI Apoptosis Detection Kit,Beyotime)). After staining in the dark for 20 min, the cells were analysed by flowcytometry (EPICS XL/XL-MCL, Beckman). The experiment was performed intriplicate and independently. Animals. Female BALB/c mice with an average age of 7 weeks (~20 g) were used forthe 4T1 tumour xenograft and corresponding assessments of tumour-starvingtherapy in vivo. Female Kunming mice with an average age of 6 weeks (~20 g) wereused to evaluate the potential toxicity of MS NPs through i.v. administration. All themice were purchased from the Shanghai Laboratory Animal Center, ChineseAcademy of Sciences. All the animal experiments were performed in accordancewith the protocols approved by the Institutional Animal Care and Use Committeeand the care regulations approved by the administrative committee of laboratoryanimals of Fudan University. Animals were randomly allocated and five-group-housed per cage with a reversed 12 h light/dark cycle. Tumour models. A transplantable mouse 4T1 breast tumour model with a hightumorigenicity and invasion was used in this study48. BALB/c mice weresubcutaneously inoculated in the thigh with 1×10°4T1 cells suspended in 100 ul ofPBS, and further fed until the tumour volume reached 100-130 mm’(10 d aftertumour inoculation) to prepare the subcutaneous 4T1 xenografted tumour model.Bilateral and unilateral 4T1 xenografted-tumour-bearing BALB/c mice were used forthe tumour-starving therapy through i.t. and i.v. DOA administrations, respectively. Evaluation of the in vivo anticancer effect. The right tumours of bilateral 4T1xenografted tumour-bearing BALB/c mice were intratumorally injected with the MSNPs (1 M in 30 pl saline) and equivoluminal saline was injected into the left one asthe control. Additionally, the tumours injected with the mixture of Mg’and SiOzmicrosheets originated from the same-dose MS NPs post-deoxygenation were usedin vitro as another negative control. Subsequently, the maximum length (L) andmaximum width (W) of a tumour were measured every 2 d with a digital caliper(n=5 for each group), and the tumour volume (V) was calculated as V=L×W/2The relative tumour volume (V/V) was normalized to their initial volume (Vo). Atgiven times, the intratumoral oxygen tension was measured by a Unisense oxygenmicroelectrode equipped with a hand-operated micropropeller (SupplementaryFig. 19). Tumours collected at pre-treatment and at 1,6 and 24 h and 16 dpost-treatment were fresh frozen by immersion in liquid nitrogen, dried under vacuumand vapour deposited on thin Pt for SEM and EDS analysis. Their correspondingH&E-staining tissue sections were used for pathological assay. At 1 h and 0.5, 1, 2, 3,5, 7, 11 and 15 d post-DOA injection, these corresponding tumours were collected,weighed and then digested with aqua regia for the ICP-OES analyses of the Mg andSi content. The percentage injected dose per gram (% ID g) tumour tissue (D) ofMg or Si was calculated by the following equation: where m (rg) is the mass of Mg or Si in the measured tumour of mass morgan (g), andthe constant mp (ug) is the initially injected dose of Mg or Si. When the controltumour volume reached 1,000 mm’at 16 d post-treatment, the mice wereeuthanized in consideration of the care regulations. The group assignments andtumour collections in all the in vivo experiments were random without anysubjective factor, and all the data were randomly collected but were not blinded. Asignificant positive result (anticancer effect) was observed in all the individualtumours injected with MS NPs, and no animals were excluded from the analysis.Furthermore, the calculated P value is smaller than 0.001, which indicates that thesample size of n=5 used in the in vivo experiment is adequate to confirm thepositive result. F-MISO PET/CT imaging to assess the hypoxia level in vivo. 4T1 xenograftedtumour-bearing BALB/c mice were each injected via the tail vein with 1 mCi ofF-MISO diluted in 100 ul of saline. With consecutive anaesthetization by using 2%isoflurane in oxygen, the early PET/CT (SuperNova) scanning was estimated 2 hafter the tracer injection. Then, for i.t. administration on the bilateral 4T1 tumour-bearing mice, the right tumours were intratumorally injected with MS NPs(1 M in10 pl saline; the intention of the decreased dosage of MS NPs here is to prevent anexcessive blood-capillary-blocking effect of the subsequent SiO2 microsheet formation,which may significantly suppress the circulation of 18F-MISO in the tumour) andequivoluminal saline was injected into the left ones as the control. Late PET/CTscanning was then performed 1 and 3 h after the treatments. In the case of i.v.administration of MS NPs, late PET/CT scanning was performed 3 h after thei.v. injection of MS NPs (100 mg Mg kgin 150 ul saline). The PET/CT imagingexperiment was performed on three independent bilateral 4T1 tumour-bearing mice.The corresponding ratios of late and early standard uptake value (SUV) ofF-MISO inthe tumour region were further measured and calculated with SuperNova software. Distribution of Si and Mg species in tumours. The fresh 4T1 tumours collected atgiven times of 30 min, 2 and 24h post-i.t. injection of MS NPs (1 M in 30 ul saline)were immediately frozen in liquid nitrogen to avoid further evolution of Si and Mgspecies in the tumour, then sectioned in the largest tissue area and dried undervacuum. Subsequently, the tumour sections were analysed by X Series II inductivelycoupled plasma mass spectrometry (Thermo Fisher) equipped with a LSX-213 Nd:YAG Laser Ablation System (CETAC Technologies) for pointwiseSi and 24Mgelement-content measurements. Surfer 8 software was used for the mass spectralprocessing and reconstitution of the ratiometric mapping of Si/Mg (mol) in thecorresponding tumour section. The value of the point with no detectable 2si orMg is defined as zero, shown as white in the ratiometric mapping. 29Si solid-state MAS NMR of excised tumours. The fresh 4T1 tumours collectedat given times of 30 min and 12 and 60 h post-i.t. injection of MS NPs (1 M in 30 plsaline) were immediately frozen in liquid nitrogen and dried under vacuum. Then,the Si solid-state MAS NMR spectra were recorded on a Varian Model VNMRS-400WB spectrometer under a one pulse condition and cross-polarization,respectively. The spectra were acquired with a 7.5 mm T3HX probe at 79.43 MHzand a spinning rate of 3 kHz. The chemical shift was referred to 2,2-dimethyl-2-silapentane-5-sulfonic acid sodium salt ((CH3)3Si(CH2)3SO Na). Assessment on the permeability of the intratumoral vascularity by MRI. The invivo MRI tests were performed on a 3.0 T clinical MRI instrument (GE Signa 3.0 T)equipped with a specialized small-animal coil (WK603,Magtron Inc.). Withconsecutive anaesthetization by using 2% isoflurane in oxygen, the T1-weightedMRI scanning was first performed on the bilateral 4T1 xenografted tumour-bearingmouse without any contrast administration. Then, the right tumour wasintratumorally injected with MS NPs (1M in 30 ul saline) and equivoluminal salinewas injected into the left one as the control. At 3 h post-administration, contrast-enhanced T1-weighted MRI scans were performed consecutively at 5 and 30 min afteran instant i.v. injection of Magnevist (gadopentetate dimeglumine, 0.1 M, 150 pl) viathe tail vein. The MRI was performed on three independent 4T1 tumour-bearing mice.For the coronal T1-weighted MRI, repletion time=17.4 ms, flip angle=20, slice. thickness =0.8 mm, frequency field of view (FoV)=6.0, phase FoV=1.00, frequency ×phase=352×320, number of excitations=1.00 and bandwidth=62.5. Statistical analysis. Quantitative data are expressed as mean ±s.d. Statisticalcomparisons were conducted by using Student's two-sided t-test with the resultsshown in Figures 4 and 5 as n.s. (no significance),*P<0.05 (significant), **P<0.01(moderately significant) and ***P<0.001 (highly significant). References 46. Zijlstra, W. & Buursma, A. Spectrophotometry of hemoglobin: absorptionspectra of rat oxyhemoglobin, deoxyhemoglobin, carboxyhemoglobin, andmethemoglobin. Comp. Biochem. Physiol. B 118, 743-749 (1997). 47. Zhang, C. et al. Synthesis of iron nanometallic glasses and their application incancer therapy by a localized Fenton reaction. Angew. Chem. Int. Ed. 55,2101-2106(2016). 48. Pulaski, B. A. & Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr.Protoc. Immunol.20, 1-16(2001). NATURE NANOTECHNOLOGY|ADVANCE ONLINE PUBLICATION|www.nature.com/naturenanotechnology@Macmillan Publishers Limited, part of Springer Nature. All rights reserved. 国际著名学术期刊《自然-纳米技术》(Nature Nanotechnology)近日在线发表了中科院上海硅酸盐研究所施剑林研究员课题组与华东师范大学化学与分子工程学院、上海市绿色化学与化工过程绿色化重点实验室步文博教授研究团队的研究论文“Magnesium silicide nanoparticles as a deoxygenation agent for cancer starvation therapy”(Nature Nanotechnology, 2017,12 (4) :378-387)。 该研究从采用自蔓延燃烧方法合成新型Mg2Si纳米颗粒出发,揭示了肿瘤微环境可以特异性激活Mg2Si纳米颗粒的耗氧功能和分解产物堵塞肿瘤血管的新现象,开创性提出了无机耗氧剂用于肿瘤饥饿疗法的新思路,为传统的肿瘤饥饿疗法注入了新活力。 众所周知,肿瘤在人体内生长过程中,需要形成自身的血管网络用于提供肿瘤组织生长必需的氧气分子和营养成分,因此“血管”就是肿瘤的“命门”;通过破坏或阻塞肿瘤的血管,就能切断对肿瘤组织的血供,肿瘤必然死亡,这就是哈佛大学佛克曼教授于1971年首次提出的切断肿瘤血管、饿死癌细胞的“肿瘤饥饿疗法”。 然而遗憾的是,由于“肿瘤饥饿疗法”对无机材料的苛刻要求(如满足肿瘤组织的特异性、耗氧能力强、阻塞血管持久性,可注射性、良好的生物相容性等要求),目前无机材料用于肿瘤饥饿疗法还无法实现。最近,中科院上海硅酸盐研究所施剑林研究员与华东师范大学化学与分子工程学院步文博教授研究团队合作,采用改进的自蔓延燃烧法,成功制备了单分散、直径约100nm的硅化镁 (Mg2Si) 纳米耗氧剂,经聚乙烯吡咯烷酮(PVP)表面改性后形成稳定的溶胶,赋予其良好的可注射性。图1、肿瘤饥饿疗法示意图实验结果显示,该新型耗氧剂在肿瘤组织弱酸性微环境下被特异性激活,利用中间产物硅烷(SiH4)快速高效、持久消耗肿瘤组织及血管中的溶解氧和血红蛋白结合氧,导致肿瘤病灶区极度缺氧;更重要的是,耗氧剂的分解产物SiO2在肿瘤组织血管中原位自聚集形成微米级絮状物,高效率阻塞肿瘤血管,切断肿瘤周围的氧分子和营养成分通过肿瘤血管系统的供给,同时也切断了肿瘤侵袭、转移的血管途径,致使癌细胞发生明显的纤维化、凋亡和坏死,达到“饿死肿瘤”的治疗效果;值得一提的是,该策略采用的制备工艺可以批量化、低成本制备硅化镁耗氧剂,并仅能在肿瘤病灶区特异性激活其耗氧功能,正常组织和器官中无法激活其耗氧功能(各组织及器官内的氧浓度采用unisense氧微电极实时原位测试),该类不含有重金属离子的新型耗氧剂,不但自身具有良好的生物相容性,其分解产物(Mg2+和SiO2)同样也无毒无害,并最终被安全地代谢出体外,克服了医用无机材料难降解、活体内滞留易导致生物毒性的医学难题。该工作为批量化制备新型功能纳米材料提供了新的方法,同时为“肿瘤饥饿疗法”提供了新的思路。图2实验老鼠活体组织的氧浓度测试实验研究过程中采用Unisense氧微电极OX-25原位的测试老鼠静脉血液以及老鼠肿瘤组织中的氧气浓度(图2所示),其中老鼠体内的各个器官肿瘤组织氧气浓度是通过使用氧微电极穿刺测试获到的,由于该类型的电极的端口为微米级,能够轻易的穿刺入动物的组织内。从而实现了对于实验老鼠体内肿瘤组织的氧气浓度的实时监测(图3所示),从而为进一步了解耗氧剂在肿瘤组织内发挥的作用,同时为研究人员的“肿瘤饥饿疗法”机理的提出提供了可信的测试数据。图(3)、老鼠各器官组织中的氧分压值 该研究工作以Article的形式在线发表于Nature Nanotechnology,并在最热门论文中排序第二。该工作一经发表即受到国际学术界的广泛关注,国际学术期刊《Cell》子刊《Chem》、美国化学会Chemical & Engineering News和世界科技研究新闻资讯网等多个国际权威学术期刊/新闻媒体对该研究成果进行了专题报道和亮点评述。研究工作得到了国家自然科学基金委和上海市科委学术带头人计划的支持。
关闭-
1/11

-
2/11
还剩9页未读,是否继续阅读?
继续免费阅读全文产品配置单
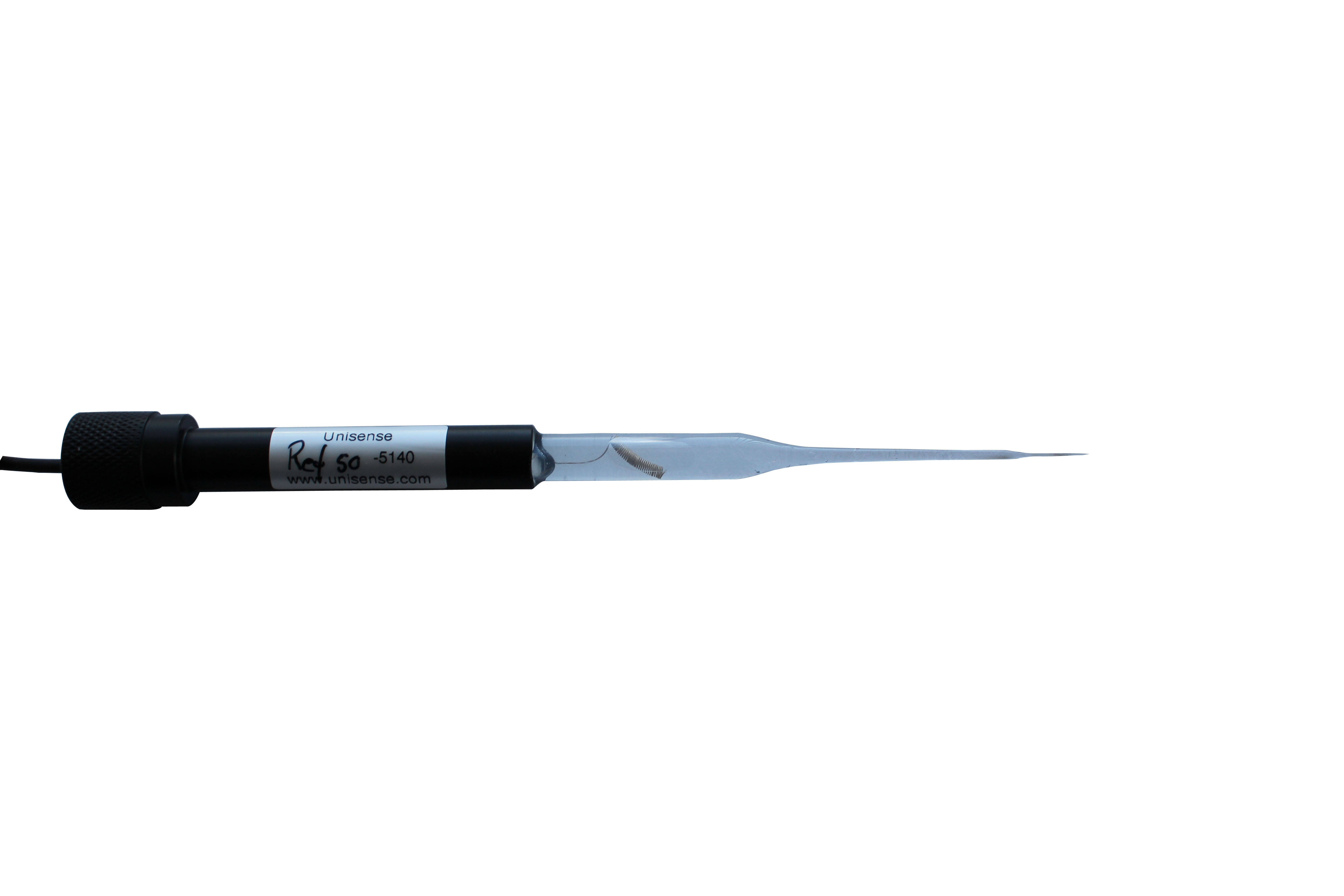
上海谓载科技有限公司为您提供《肿瘤组织、血管中血氧浓度检测方案(溶解氧测定仪)》,该方案主要用于绒毛膜组织中血氧浓度检测,参考标准《暂无》,《肿瘤组织、血管中血氧浓度检测方案(溶解氧测定仪)》用到的仪器有丹麦Unisense溶氧仪、丹麦unisense pH测量仪。
我要纠错
推荐专场
相关方案








 咨询
咨询





