方案详情文
智能文字提取功能测试中
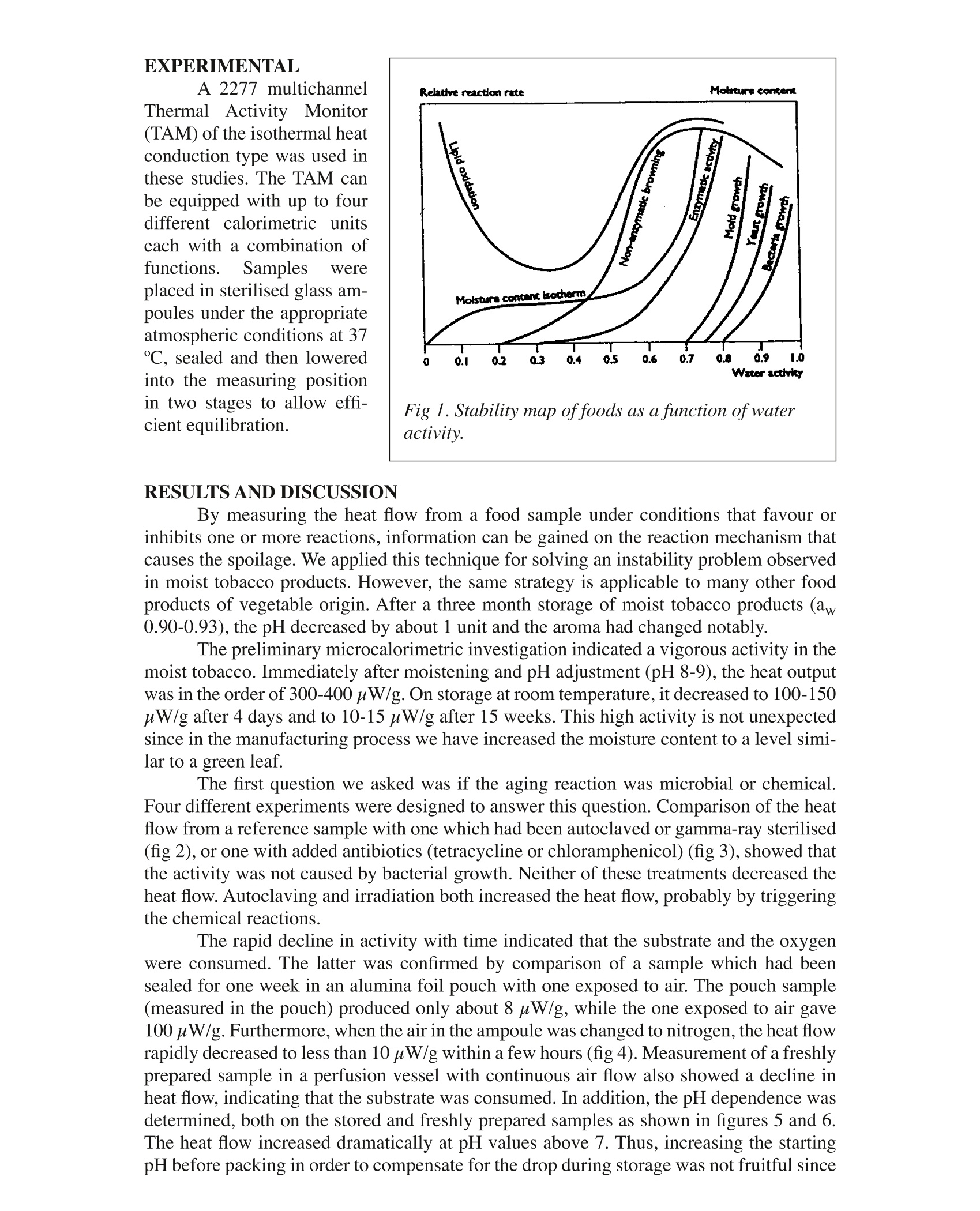
Microcalorimetry: A Useful Toolfor Solving Shelf Life Problems in the Food Industry Sven-Olof Almqvist, Lotta Karlson DunasAnn Odnevall Rorstrom, Anna Wiernik Reserca AB, 11824 Stockholm Sweden INTRODUCTION Food items are often perishable and have a limited shelf life. Legislation requiring adeclaration of “Best before"date and the interest of the manufacturer to assure the qualityof his products within the declared time makes a firm control of the adulterating reactionsin the product highly desirable, A thorough knowledge of the aging mechanism allows themanufacturer to take proper actions to prevent rapid decay. Microcalorimetry is a very useful tool for the measurement of the degree of agingof a product and also for the elucidation of the aging mechanism. This can sometimes be aformidable task in such a complex matrix as a food product. The main mechanisms for food deterioration are: Bacterial growth Mould or yeast growth Enzymatic oxidation reactions Chemical oxidation reactions Hydrolylic reactions Condensation reactions like the Milliard browning reaction. The degree of instability of food products is determined by its water activity (aw)(Ref 1). Approximate water activities and the expected shelf life for some common foodsare shown in table 1. Labuza et al (Ref2) showed that the water activity to a great degreedetermines the reaction rate of many spoilage reactions as shown in fig 1. We usually ex-pect a dry food like biscuits to last longer than moist foods like fresh vegetables and meat,since most of the spoilage reactions are favoured by a high aw. Bacterial growth requires ahigh aw value, typically 0.90. However, different bacterial types have different limits forgrowth (Ref3). Food Item Approx aw Expected shelf life Fruit, Vegetable, 0.99 Week Milk, Fish, Meat Bread 0.98-0.93 Week Sausage, ham, jam 0.96-0.93 Month Marmalade 0.87-0.60 Month-Year Dried fruit 0.60 Year Biscuits <0.50 Year Table 1. Water activity and the expected shelf life for a variety offoods. EXPERIMENTAL A 2277 multichannelThermail AActivity Monitor(TAM) of the isothermal heatconduction type was used inthese studies. The TAM canbe equipped with up to fourdifferent calorimetric unitseach with a combination offunctions.Samples wereplaced in sterilised glass am-poules under the appropriateatmospheric conditions at 37C, sealed and then loweredinto the measuring positionin two stages to allow effi-cient equilibration. Fig 1. Stability map of foods as a function of wateractivity. By measuring the heat flow from a food sample under conditions that favour orinhibits one or more reactions, information can be gained on the reaction mechanism thatcauses the spoilage. We applied this technique for solving an instability problem observedin moist tobacco products. However, the same strategy is applicable to many other foodproducts of vegetable origin. After a three month storage of moist tobacco products (aw0.90-0.93), the pH decreased by about 1 unit and the aroma had changed notably. The preliminary microcalorimetric investigation indicated a vigorous activity in themoist tobacco. Immediately after moistening and pH adjustment (pH 8-9), the heat outputwas in the order of 300-400uW/g. On storage at room temperature, it decreased to 100-150uW/g after 4 days and to 10-15 uW/g after 15 weeks. This high activity is not unexpectedsince in the manufacturing process we have increased the moisture content to a level simi-lar to a green leaf. The first question we asked was if the aging reaction was microbial or chemical.Four different experiments were designed to answer this question. Comparison of the heatflow from a reference sample with one which had been autoclaved or gamma-ray sterilised(fig 2), or one with added antibiotics (tetracycline or chloramphenicol) (fig 3), showed thatthe activity was not caused by bacterial growth. Neither of these treatments decreased theheat flow. Autoclaving and irradiation both increased the heat flow, probably by triggeringthe chemical reactions. The rapid decline in activity with time indicated that the substrate and the oxygenwere consumed. The latter was confirmed by comparison of a sample which had beensealed for one week in an alumina foil pouch with one exposed to air. The pouch sample(measured in the pouch) produced only about 8 uW/g, while the one exposed to air gave100 uW/g. Furthermore, when the air in the ampoule was changed to nitrogen, the heat flowrapidly decreased to less than 10 pW/g within a few hours (fig 4). Measurement of a freshlyprepared sample in a perfusion vessel with continuous air flow also showed a decline inheat flow, indicating that the substrate was consumed. In addition, the pH dependence wasdetermined, both on the stored and freshly prepared samples as shown in figures 5 and 6.The heat flow increased dramatically at pH values above 7. Thus, increasing the startingpH before packing in order to compensate for the drop during storage was not fruitful since this increased the oxidationprocess. Oxygen consump-tion was measured in sepa-rate experiments using sealedalumina foil bags. The aver-age hourly oxygen consump-tion during the first day afterpacking was about 1ml/100gof tobacco. The rate wastwice as high in the first fewhours. Thus, in a sealed air-tight package, all availableoxygen is consumed withinthe first three days. The main reaction isa base catalysed oxidationreaction. Chemical analysisof fresh and stored tobaccosamples showed a large de-crease in the amounts of phe-nols having a catechol or py-rogallol structure on storage. Thus. chlorogenicacid and rutin, major poly-phenols in many plants werecompletelyabsentin thestored samples. The contentof other substances like guia-col and syringol and their al-kyl substituted homologuesdecreased to less than onetenth of the original value af-ter only 4 days at room tem-perature.These substancesproduced by wood smoke,were deposited on tobaccoduring the “smoke fire-cur-ing”applied to some of thetobacco types used in mak-ing the product. Revieowf the lit-erature showed that lignine, Fig 2. Heat flow curves ofautoclaved and irradiatedtobacco samples. Fig 3. Heat flow curves ofantibiotic sterilised tobac-co samples. present in plant material, was easily oxidised by air in alkaline solution (Ref 4). Lignine isconsidered to be built from phenols of the guiacol and syringol type. Lignine, while beingoxidised produces a drop of pH. The mechanism for this reaction has been elucidated fora number of model substances which make up the backbone of the polymer (Ref 5). Theformation of a substituted benzoic acid and carbon dioxide explain the drop in pH. The obvious solution to the instability problem is to chose an airtight package,possibly also using an inert atmosphere like nitrogen. The microcalorimetric results alsodemonstrated the importance of storing the product at low temperatures. The reaction rate increases 10-fold for a 20°aCrise in temperature. In conclusion thefirst rapid reaction is due tothe oxidation of monomericsoluble phenols, while theslower reaction is caused byoxidation of insoluble poly-meric material like lignine.This was confirmed by theobservation of substantial-heat generation from the solidmatrix remaining after waterextraction of the tobacco. It is well known thatthe oxidation of polyphe-nols, guiacol and syringol isalso catalysed by the plantenzymes polyphenoloxidaseandpolyphenolperoxidase.These enzymes are also pres-ent in tobacco and have op-timum activity at about pH6. The fairly constant rateobserved in the pH range 5-7(fig 6) and the steep increaseat pH 7 suggests that the en-zymatic reaction dominatesat the lower pH range and thepure chemical base catalysedreaction dominates at higherpH. The same strategy asdescribed above was appliedto another moisttobaccoproduct. In this case, the in-stability problem was due togrowth of bacteria requiringrelatively low water activ-ity. Addition of tetracyclinelowered the heat output by3-5 p W/g. By adding small Fig 4. Heat flow curves of tobacco samples, under dif-ferent atmospheric conditions. Fig 5. Heat flow curves of tobacco samples at differ-ent pH. amounts of water to increase the aw by 0.01 units, bacterial growth produced a 40-50 uWhigher heat flow after a few days than the sample with slightly lower aw. The tobacco wasfound to have an avalue close to the growth limit for the resident bacterial flora. Although our work was focused on tobacco products, similar aging problems are ex-pected to occur in other food products. Polyphenols are ubiquitous in plant material. Plantsalso contain other substances with similar structure, for example Con-iferylalcohol, Sina-pylalcohol, Ferulic and Syringa acids. Smoke flavour phenols are present in smoked fishand meat products. Even spices like clove contain easily oxidised phenols like Eugenol. REFERENCES 1. Fuchs, G., Compendium of Food Chemistry, Statens Livsmedelsverk, Uppsala,Sweden (1982). 2 Labuza, T. A. McNally, L. Gallaghar, D. Hawkes, J. Hurtado, F.J.J. Food Science37:154(1972). 3. Leistner, L. Rodel, W. Krispien,K.“Microbiology of Meat and Meat products inHigh and Intermediate Moisture Ranges". In Water Activity: lnfluences on FoodQuality. Ed:Rockland and Stewart, Academic Press (1981). 4Raff,R.A.F. Tomlinson,G.H. Canadian Journal of Research 27:399 (1949). 5 Kratzl, K. Gratzl, J. Advances in Chemistry Series 59 (1969). Food items are often perishable and have a limited shelf life. Legislation requiring a declaration of “Best before” date and the interest of the manufacturer to assure the quality of his products within the declared time makes a frm control of the adulterating reactions in the product highly desirable, A thorough knowledge of the aging mechanism allows the manufacturer to take proper actions to prevent rapid decay. Microcalorimetry is a very useful tool for the measurement of the degree of aging of a product and also for the elucidation of the aging mechanism. This can sometimes be a formidable task in such a complex matrix as a food product.
关闭-
1/5

-
2/5
还剩3页未读,是否继续阅读?
继续免费阅读全文产品配置单
TA仪器为您提供《食品中食品保质期检测方案(差示扫描量热)》,该方案主要用于小麦粉中营养成分检测,参考标准《暂无》,《食品中食品保质期检测方案(差示扫描量热)》用到的仪器有TA仪器+等温量热仪+TAM Air。
我要纠错
推荐专场
差示扫描量热仪(DSC/DTA)
更多相关方案






 咨询
咨询





