方案详情文
智能文字提取功能测试中
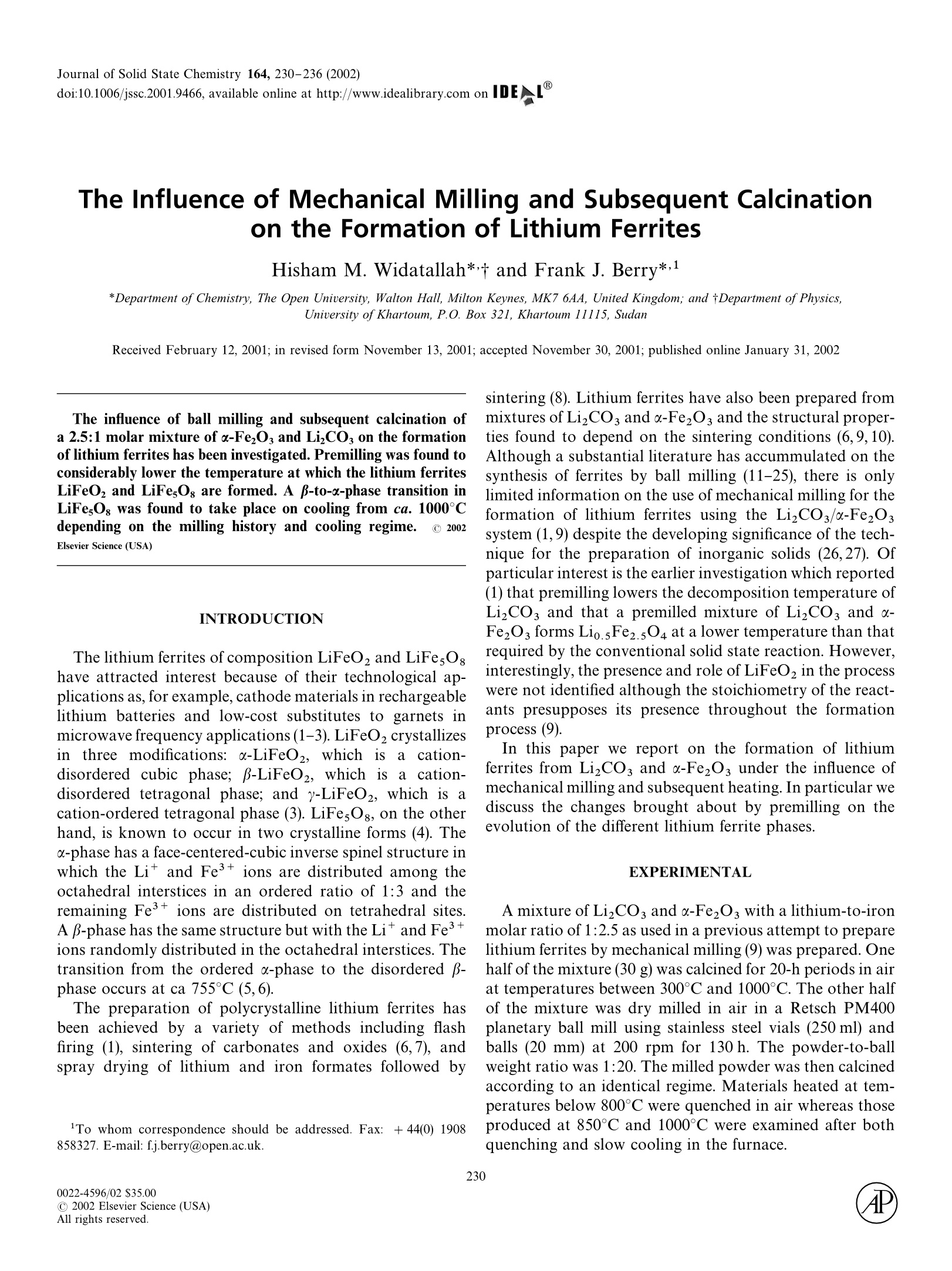
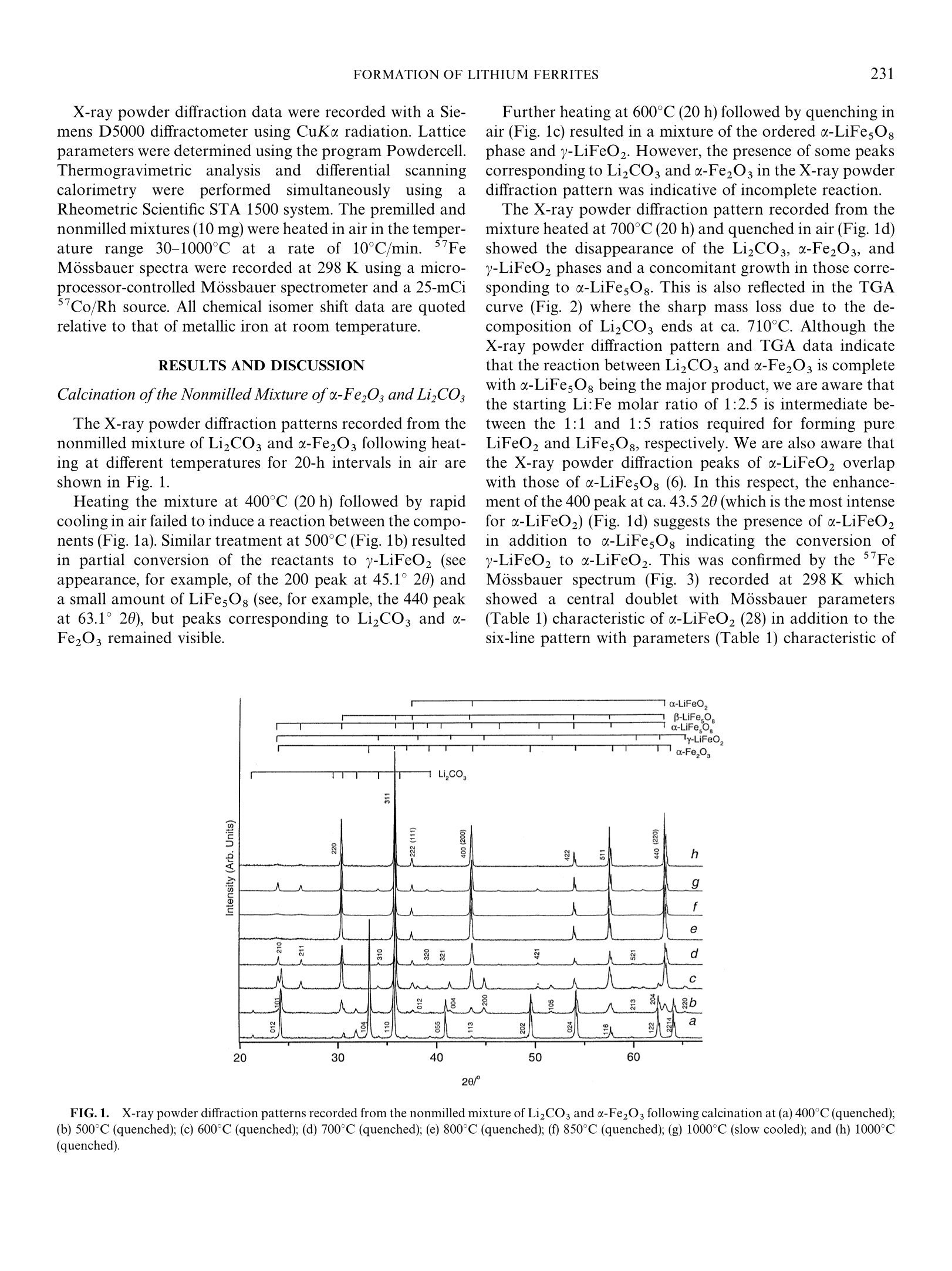
Journal of Solid State Chemistry 164, 230-236 (2002)doi:10.1006/jssc.2001.9466, available online at http://www.idealibrary.com on IDEAL 231FORMATION OF LITHIUM FERRITES 2304P0022-4596/02 $35.00C 2002 Elsevier Science (USA)All rights reserved. The Influence of Mechanical Milling and Subsequent Calcinationon the Formation of Lithium Ferrites Hisham M. Widatallah* and Frank J. Berry*,l *Department of Chemistry, The Open University, Walton Hall, Milton Keynes, MK7 6AA, United Kingdom; and tDepartment of Physics,University of Khartoum, P.O. Box 321, Khartoum 11115, Sudan Received February 12, 2001; in revised form November 13, 2001; accepted November 30, 2001; published online January 31, 2002 The influence of ball milling and subsequent calcination ofa 2.5:1 molar mixture of o-FezOs and LizCOs on the formationof lithium ferrites has been investigated. Premilling was found toconsiderably lower the temperature at which the lithium ferritesLiFeOz and LiFe,Og are formed. A B-to-o-phase transition inLiFesOs was found to take place on cooling from ca. 1000°Cdepending on the milling history and cooling regime. C 2002Elsevier Science (USA) INTRODUCTION The lithium ferrites of composition LiFeO, and LiFesOghave attracted interest because of their technological ap-plications as, for example, cathode materials in rechargeablelithium batteries and low-cost substitutes to garnets inmicrowave frequency applications (1-3). LiFeO2 crystallizesin three modifications: a-LiFeO2, whichi1Sacation-disordered cubic phase; B-LiFeO2, which isa cation-disordered tetragonal phase; and y-LiFeO2, which is acation-ordered tetragonal phase (3). LiFes Og, on the otherhand, is known to occur in two crystalline forms (4). Theo-phase has a face-centered-cubic inverse spinel structure inwhich the Li and Fe3+ ions are distributed among theoctahedral interstices in an ordered ratio of 1:3 and theremaining Fe3+ ions are distributed on tetrahedral sites.A B-phase has the same structure but with the Liand Fe3+ions randomly distributed in the octahedral interstices. Thetransition from the ordered o-phase to the disordered61 -phase occurs at ca 755°℃ (5,6). The preparation of polycrystalline lithium ferrites hasbeen achieved by a variety of methods including flashfiring (1), sintering of carbonates and oxides (6,7), andspray drying of lithium and iron formates followed by ( To whom correspondence should b e a d dressed. F ax: + 44(0) 1908858327. E -mail: f.j.berry@open.ac.uk. ) sintering (8). Lithium ferrites have also been prepared frommixtures of Li2CO and o-Fe2O3 and the structural proper-ties found to depend on the sintering conditions (6,9,10).Although a substantial literature has accummulated on thesynthesis of ferrites by ball milling (11-25), there is onlylimited information on the use of mechanical milling for theformation of lithium ferrites using the LizCO3/u-Fe2O3system (1,9) despite the developing significance of the tech-nique for the preparation of inorganic solids (26,27). Ofparticular interest is the earlier investigation which reported(1) that premilling lowers the decomposition temperature ofLizCOs and that a premilled mixture of LizCOs and a-Fe2O3 forms Lio.5Fe2.5O4 at a lower temperature than thatrequired by the conventional solid state reaction. However,interestingly, the presence and role of LiFeO2 in the processwere not identified although the stoichiometry of the react-ants presupposes its presence throughout the formationprocess (9). In this paper we report on the formation of lithiumferrites from LizCOs and -FezOs under the influence ofmechanical milling and subsequent heating. In particular wediscuss the changes brought about by premilling on theevolution of the different lithium ferrite phases. EXPERIMENTAL A mixture of LizCO3 and u-Fe2O3 with a lithium-to-ironmolar ratio of 1:2.5 as used in a previous attempt to preparelithium ferrites by mechanical milling (9) was prepared. Onehalf of the mixture (30 g) was calcined for 20-h periods in airat temperatures between 300°C and 1000°C. The other halfof the mixture was dry milled in air in a Retsch PM400planetary ball mill using stainless steel vials (250 ml) andballs (20 mm) at 200 rpm for 130 h. The powder-to-ballweight ratio was 1:20. The milled powder was then calcinedaccording to an identical regime. Materials heated at tem-peratures below 800℃ were quenched in air whereas thoseproduced at 850°C and 1000°C were examined after bothquenching and slow cooling in the furnace. X-ray powder diffraction data were recorded with a Sie-mens D5000 diffractometer using CuKu radiation. Latticeparameters were determined using the program Powdercell.Thermogravimetric analysisand differentialilscanningcalorimetrywere performed ssimultaneouslyy uusingaRheometric Scientific STA 1500 system. The premilled andnonmilled mixtures (10 mg) were heated in air in the temper-ature range 30-1000℃ at a rate of 10℃/min. 57FeMossbauer spectra were recorded at 298 K using a micro-processor-controlled Mossbauer spectrometer and a 25-mCiCo/Rh source. All chemical isomer shift data are quotedrelative to that of metallic iron at room temperature. RESULTS AND DISCUSSION Calcination of the Nonmilled Mixture ofu-FezO and LizCO; The X-ray powder diffraction patterns recorded from thenonmilled mixture of LizCOs and u-Fe2O3 following heat-ing at different temperatures for 20-h intervals in air areshown in Fig. 1. Heating the mixture at 400℃ (20 h) followed by rapidcooling in air failed to induce a reaction between the compo-nents (Fig. 1a). Similar treatment at 500℃(Fig.1b) resultedin partial conversion of the reactants to y-LiFeO2 (seeappearance, for example, of the 200 peak at 45.1°20) anda small amount of LiFesO: (see, for example,the 440 peakat 63.1°20), but peaks corresponding to LizCOs and a-Fe2Os remained visible. Further heating at 600°℃ (20 h) followed by quenching inair (Fig. 1c) resulted in a mixture of the ordered a-LiFesO8phase and y-LiFeO2. However, the presence of some peakscorresponding to LizCO3 and a-Fe2O3 in the X-ray powderdiffraction pattern was indicative of incomplete reaction. The X-ray powder diffraction pattern recorded from themixture heated at 700℃(20 h) and quenched in air (Fig.1d)showed the disappearance of the LizCO3, a-Fe2O3, andy-LiFeO2 phases and a concomitant growth in those corre-sponding to u-LiFesO8. This is also reflected in the TGAcurve (Fig. 2) where the sharp mass loss due to the de-composition of LizCO, ends at ca. 710°C. Although theX-ray powder diffraction pattern and TGA data indicatethat the reaction between LizCO3 and o-FezO3 is completewith a-LiFesOg being the major product, we are aware thatthe starting Li:Fe molar ratio of 1:2.5 is intermediate be-tween the 1:1 and 1:5 ratios required for forming pureLiFeO2 and LiFesO, respectively. We are also aware thatthe X-ray powder diffraction peaks of u-LiFeO2 overlapwith those of a-LiFesO8 (6). In this respect, the enhance-ment of the 400 peak at ca. 43.520 (which is the most intensefor a-LiFeO2) (Fig. 1d) suggests the presence of a-LiFeO,in addition to a-LiFesOg indicating the conversion ofy-LiFeO to u-LiFeO. This was confirmed by the 57FeMossbauer spectrum (Fig. 3) recorded at 298 K whichshowed aa cceentral doublet with Mossbauer parameters(Table 1) characteristic of α-LiFeO2 (28) in addition to thesix-line pattern with parameters (Table 1) characteristic of 20/° FIG.1.. X-raypowder diffraction patterns recorded from the nonmilled mixture of LizCO3 and o-Fe2O3 following calcination at(a) 400°℃ (quenched);(b) 500℃ (quenched);(c) 600C (quenched); (d) 700℃ (quenched); (e) 800°℃ (quenched); (f) 850℃ (quenched); (g) 1000℃ (slow cooled); and (h) 1000℃(quenched). Temperature/℃ FIG.2. TGA (solid line) and DSC (dashed line) recorded from the nonmilled mixture of LizCO3 and o-FezO3. the spinel-related a-LiFesO: phase (5,7,29,30). We adopteda simple fitting model (29) to fit the Fe Mossbauer spec-trum of a-LiFesOg with two overlapping sextets, A and B,for the octahedral and tetrahedral sites with areas propor-tional to the 3:2 occupancy of the Fe+ions. Assuming thesame recoilless fraction for a-LiFesOg and a-LiFeO, therelative amount of iron in each phase determined from theMossbauer spectrum is 3:1. The reaction stoichiometrymay, therefore, be described as which reflects both the 2.5:1 molar ratio of the reactants andthe abundance ofeach phase in the products. FIG.3.5Fe Mossbauer spectrum recorded from the nonmilled mix-ture of LizCOs and -Fe2O following calcination at 700℃ (20 h) andquenching. The X-ray powder diffraction pattern recorded from themixture after heating at 800℃ (20h) and quenching in air(Fig. 1e) showed the absence of the 210 and 211 peaks at 24and 26.2°20 and the transformation of most of the orderedo-LiFesOs phase to disordered B-LiFesOg. The a-LiFeO2phase remained unchanged at 800C. X-ray powder diffrac-tion and 57Fe Mossbauer spectroscopy showed that nofurther changes in either the B-LiFesOg or o-LiFeO2 phaseswere induced by heating at 850℃ (20 h) and quenching inair or slowly cooling in the furnace (Fig. 1f, Table 1). The X-ray powder diffraction pattern recorded from themixture following heating at 1000℃ (20 h) and slow coolingin the furnace showed a transformation of the disordered B-polymorph of LiFesOg to the ordered u-LiFe5Og form. Wewould comment that no such phase transition was observedwhen the mixture was quenched in air following calcinationat 1000°℃ (20 h) (Fig. 1h) and the result demonstrates, asmight be expected, that the cooling regime is an importantfactor in establishing the occurrence of a specific phase. TheLiFesOg-to-LiFeO, abundance ratio remained unchanged(Table 1) following cooling from 1000°C. Mechanical Milling of the Mixtureofa-Fe2O and LizCO The X-ray powder diffraction patterns recorded from themixture of LizCOs and u-FeOs following ball milling atroom temperature for different time intervals are collectedin Fig. 4. The results show that after 40 h of milling (Fig. 4b) thepeaks characteristic of LizCOs decrease in intensity andthose attributable to u-Fe2O3 begin to broaden. The crys-tallite size of the o-FezO3 phase determined from the X-ray TABLE 1 57Fe Mossbauer Parameters Recorded at 298 K from the Nonmilled Mixture of a-FezOs and LizCOsFollowing Calcination in Air Calcination )+ 0.02 A±0.04 H ± 0.3 I±0.02 Area ±3 Conditions Phase Subspectrum (mms-) (mms) (T) (mm s) (%) 700°C (20h) a-LiFesO Sextet A 0.23 0.00 50.1 0.30 29 (quenched) u-LiFesO: Sextet B 0.39 0.01 50.7 0.30 46 u-LiFeO, Doublet 0.37 0.64 0.40 25 850°C (20 h) B-LiFesO Sextet A 0.23 0.00 50.1 0.33 34 (quenched) B-LiFesO: Sextet B 0.40 0.02 50.6 0.31 41 u-LiFeO, Doublet 0.37 0.63 0.41 25 1000°C (20 h) x-LiFesO; Sextet A 0.24 0.00 50.0 0.33 35 (slow cooled) a-LiFesO: Sextet B 0.40 0.02 50.5 0.30 40 a-LiFeO, Doublet 0.37 0.63 0.40 25 powder diffraction data using the Scherrer method (Table 2)decreased from ca. 1.155 um to ca. 20 nm after 40h ofmilling and underwent no further change in crystallite sizeon subsequent milling treatment. The X-ray powder diffrac-tion pattern showed the intensity of the LizCO3 peaks todecrease after milling for 100 h and 130 h (Figs. 4c and 4d).The intensities of the 104 and 110 reflections of u-Fe2O3(atca. 33°and 36°20) changed in a way similar to that observedwhen tin is incorporated within the a-FeO3 (31). Further-more, the a-FeO3 unit cell parameters for the materialmilled for 130 h were found to decrease (Table 3) in a waysimilar to that observed when lithium is incorporated withinthe corundum-related matrix by chemical methods (32).Taken together these results indicate that milling inducesthe progressive incorporation of lithium within the nanoc-rystalline a-FezO3 structure. Since both LizCO3 and a-Fe2O3 are different in their mechanical properties (33), the small crystallite size (ca. 20 nm) inferred from the X-raypowder diffraction data and the nearly complete disappear-ance of peaks characteristic of LizCOs suggests theformation of nanoaggregates consisting of lithium-dopedu-FezO3 together with particles where both reactants enjoya large interface and are tightly pressed together. Calcination of the Premilled Mixture ofo-Fe2Os and LizCO, The X-ray powder diffraction patterns recorded from themixture of Li,CO3 and a-FezO3 milled for 130 h and fol-lowing calcination treatment similar to that used for thenonmilled mixture are shown in Fig. 5. a Heating the premilled mixture at 400°C for 20 h followedby quenching in air (Fig. 5a) resulted in the disappearance ofall the peaks corresponding to LizCO3. New peaks corre-sponding to y-LiFeO2 appeared. The intensities of the peaks a-Fe, FIG. 4. X-ray powder diffraction patterns recorded from the mixture of LizCO, and o-FezOs milled for (a) 0 h; (b) 40 h; (c) 100 h; and (d) 130h. Milling time (h) Particle size (nm) 0 1155 20 19 20 corresponding to the 104 and 110 reflections of u-Fe2O3 atca 33° and 36°20 reverted to those expected for purea-Fe2O3 with lattice parameters also characteristic of thepure material (Table 3). The results suggest that mild ther-mal treatment converts lithiated a-Fe,O3 to y-LiFeO2 aswell as initiating a reaction between unreacted LizCO3andsome a-FeO3 to form y-LiFeO2. This is endorsed by theTGA curve (Fig. 6) recorded from the premilled mixturewhich shows an initial gradual mass loss due to the de-composition of LizCO3 with increasing temperature andwhich dramatically increases at ca. 360°℃. A comparison ofthese results with those obtained from the nonmilled mix-ture shows that premilling lowers the decomposition tem-perature of LizCOs in the mixture thereby leading to theformation of y-LiFeO2 at 400°C as opposed to ca. 500Cwhen the mixture is not premilled. The influence of milling on the subsequent reaction of thepremilled powder at higher temperatures is also illustratedby the X-ray powder diffraction data recorded following 0 25 5.034 5.034 13.763 40 25 5.038 5.038 13.771 100 25 5.037 5.037 13.775 130 25 5.012 5.012 13.704 130 400 5.032 5.032 13.755 treatment at 500°C and quenching in air (Figs. 5b). Thepattern corresponded to the formation of spinel-relatedLiFesOg together with a small amount of y-LiFeO2. Nopeaks corresponding to a-Fe2O3 and Li2CO3 could beidentified, indicating that the reaction is complete at thistemperature. However, the identification of the nature of theLiFesO8 phase is difficult since the X-ray powder diffractionpeaks (Fig. 5b) are broad, thus precluding identification ofany of the weak superstructure peaks. The decomposition ofLizCO, and formation of the lithium ferrites was also dem-onstrated by the TGA curve which showed the sharp massloss due to the decomposition of Li,CO to be completed atca. 500℃ and to be accompanied by a sharp and deependothermic peak (Tmax ≈ 460℃) in the correspondingDSC curve (Fig. 6). Comparing these results with those c一 20/° FIG.5.).X-ray powder diffraction patterns recorded from the mixture of LizCO, and o-FezO3 premilled for 130 h following calcination at (a) 400℃(quenched); (b) 500℃ (quenched); (c) 600℃ (quenched); (d) 700℃(quenched); (e) 800°℃ (quenched); (f) 850℃ (quenched); (g) 1000℃(slow cooled); and (h)1000℃ (quenched). obtained from the nonmilled mixture, where the disappear-ance of X-ray powder diffraction peaks corresponding tothe reactants was achieved only after calcination at ca.700°C, shows that premilling lowers by ca. 200°C the tem-perature at which the reaction is complete and lithiumferrites are formed. X-ray powder diffraction (Fig. 5c) showed that followingfurther treatment at 600°℃ and rapid quenching, the pre-milled mixture was converted into a mixture of ordereda-LiFesOg and y-LiFeO2. Further heating to 700℃ (20 h)and quenching in air resulted in the disappearance of thepeaks corresponding to the y-LiFeO2 phase (Fig. 5d). TheFe Mossbauer spectrum (Table 4) showed the y-LiFeO2to transform to the o-LiFeO2 polymorph with the relativeamount of iron in the o-LiFesOg and u-LiFeO2 phasesbeing present in the expected 3:1 ratio. This result is at variance with results previously reported (9) where only LiFesO was reported to form under identical conditions.Further heating of the premilled mixture at 800°C and850℃ (20 h) followed by quenching gave X-ray powderdiffraction patterns (Figs.5e and 5f) that corresponded todisordered B-LiFesO and a small amount of a-LiFesO8.The DSC curve (Fig.6) showed a sharp endothermic peak atca. 755°℃ marking the order-disorder o-B phase transitionin LiFesOg as found for the nonmilled mixture. No signifi-cant difference was observed in the X-ray powder diffractionpatterns when the product was formed by slow cooling inthe furnace. The XRD pattern of the premilled mixture heated at1000℃(20 h) and subsequently slowly cooled in the furnace(Fig. 5g) showed a phase transition to ordered o-LiFesOgsimilar to that observed unexpectedly for the nonmilled TABLE 4 Fe Mossbauer Parameters Recorded at 298 K from the Premilled Mixture of a-FeOs and LizCO, Following Calcination in Air Calcination 8±0.02 A±0.04 H ±0.3 T±0.02 Area ±3 conditions Phase Subspectrum (mmsl) (mms-1) (T) (mms-1) (%) 700°C (20h) u-LiFesOg Sextet A 0.27 0.00 49.6 0.31 30 (quenched) c-LiFesOs Sextet B 0.36 0.01 50.7 0.30 45 a-LiFeO, Doublet 0.37 0.63 0.42 25 850°C (20 h) B-LiFe5O Sextet A 0.27 0.01 49.6 0.33 33 (quenched) B-LiFesO: Sextet B 0.36 0.01 50.7 0.31 42 u-LiFeO2 Doublet 0.37 0.63 0.43 25 1000°C (20 h) a-LiFesO Sextet A 0.27 0.01 49.6 0.33 32 (slow cooled) a-LiFesOs Sextet B 0.36 0.01 50.6 0.31 43 u-LiFeO2 Doublet 0.37 0.63 0.41 25 mixture. The room temperature Mossbauer parameters ofsamples prepared between700°C and 1000°C were allsimilar (Table 4). When the premilled mixture was quenchedfrom 1000℃ (20 h) in air, the X-ray powder diffractionpattern (Fig. 5h) also showed a significant partial disorder-order B-a phase transition in LiFesOg resulting in a signifi-cant amount of a-LiFesOg. This is in contrast with theX-ray powder diffraction pattern of the nonmilled mixture(Fig. 1h) where only a small amount of a-LiFesOg wasobserved after similar treatment. This suggests that thepremilling of the reactants is important in inducing a dis-order-order phase transition in LiFesOg following heattreatment at 1000°C(20h). CONCLUSION Premilling a 2.5:1 molar mixture of a-Fe2O3 and LizCO3induces the incorporation of lithium within the nanocrys-talline a-FezO3 and increases the interface between thereactants, leading to the formation of lithium ferrites atlower temperatures. y-LiFeO2 was found to form at ca.400°C compared with ca. 500°C when the mixture was notpremilled. The reaction to form lithium ferrites LiFesOgand LiFeO,from a milled mixture of reactants was found tobe complete at ca. 500°C as compared to ca. 700°C when themixture was not premilled.A β-to-a-disorder-to-orderphase transition in LiFes Og was found to take place in boththe nonmilled and premilled cases when the sample wasslowly cooled from 1000℃. The premilled reactants werealso found to undergo a partial but significant p-to-o-disorder-to-order phase transition in LiFesOg at 1000°Cwhen the sample was quenched from 1000℃. ACKNOWLEDGMENTS We thank Dr. V. Berbenni of the University of Pavia (Italy) for usefuldiscussions. We also thank the Gordon Memorial College Trust Fund, theSwedish International Co-operation and Development Agency (SIDA),and the Abdus Salam ICTP for financial and research support to H.M.W. REFERENCES ( 1 . A. J. Pointon and R . C . S a ull, J . Am. Ceram. Soc. 52, 15 7( 1969). ) ( 2. G. M. Argentina and P. D. Baba, IEEE Trans. Microwave Theory T ech. 2 2, 6 52 (1974). ) ( 3. M. T abuchi, K . Ado, H. Sakaebe, C. Masquelier, H. K a geyama, andO. N akamura, Solid State Ionics 79,220 (1995). ) ( 4. M . Schieber, J. Inorg. N ucl. C hem. 26, 1363 (1964). ) 5. J. L.Dormann, A. Tomas, and M. Nogues, Phys. Stat. Sol. (a) 77, 611(1988). ( 6. V . B erbenni, A . M ariniand, and D . Capsoni, Z. Naturforsch. (a) 53,997(1998). ) ( 7 . N . R amachandran and A. B . B iswas, J. Solid State Chem. 30, 61 ( 1979). ) ( 8. G. B onsdorf, H. L angbein, a nd K. Kense, M a ter. Res. Bull. 30, 175(1995). ) 9. J. S. Jiang, L. Gao, J. K. Guo, X. L. Yang, and H. 1. Shen, J. Inorg.Mater. (China) 14,390 (1999). ( 10. G. A. El-shokabi and A. A. Ibrahim, Thermochim. Acta 118, 15 1 (1987). ) 11. C. N. Chinnasamy, A. Narayanasamy, N. Ponpandian, and K.Chattopadhyay, Mater. Sci. Eng. A 304-305,983 (2001). ( 12. C. Mendoza-Suarez, J. A . M atutes-Aquino, J. I . E scalante-Garcia, H. M archa-Molinar, D. Rios-Jara, a n d K. K. Jo h l, J. M a gn. Ma g n. Mater.2 2 3, 55 (2001). ) 13. J. S. Jiang, L. Gao, X. L. Yang, J. K. Guo, and H. L. Shen, Mater.Sci. Lett. 203, 141(1999). 14. D. Arcos, R. Valenzuela, M. Vazquez, and M. Vallet-Regi, J. Magn.Magn. Mater. 196-197, 173 (1999). 15. S. F. Moustafa and M. B. Morsi, Mater. Lett. 34, 241 (1998). 16. G. F. Goya and R. Rechenberg, J. Magn. Magn. Mater. 203,141 (1999). 17. N. Millot, S. Begin-Colin, P. Perriat, La Caer, and B. Malaman,Nanostruct. Mater. 12, 641 (1999). 18. M. H. Mahmoud, H. H. Hamdeh, J. C. Ho, M. J. O'Shea, andJ. C. Walker, J. Magn. Magn. Mater. 220, 139 (2000). 19. C. N. Chinnasamy, A. Narayanasamy, N. Ponpandian, and K.Chattopadhyay, H. Guerault, J-M Grenech, J. Phys. CondensedMatter 12, 7795 (2000). 20. H. Yamamoto and D. Gaku, J. Jpn. Soc. Powder Powder Metallurgy47,160 (2001). 21. G. F. Goya, R. Rechenberg, and J. Z. Jiang. Mater. Sci. Forum312, 545 (1999). 22. J. Z. Jiang, L. Gerward, and S. Morup, Mater. Sci. Forum 312, 115(1999). 23. W. A. Kaczmarek, J. Magn. Magn. Mater.196, 173 (1999). ( 24. S. F. Moustafa a n d M. B. Mo r si, Mater. Let t . 34, 241 (1998). ) 25. E. Wu, S. J. Campbell, and W. A. Kaczmarek, J. Magn. Magn. Mater.177,255(1998). 26. A. Arcos, R. Valenzuela, M. Vazquez, and M Vallet-Regi, J. SolidState Chem. 141, 10 (1998). 27. S. Begin-Colin, T. Girot, G. L. Caer, and A. Macellin, J. Solid StateChem.149,41(2000). 28. D. E. Cox, G Shirane, P. A. Flinn, S. L. Ruby, W. J. Takei, Phys.Rev.132,1547(1963). 29. M. V. Kuznetsov, Q. A. Pankhurst, and I. P. Parkin, J. Phys. D:Appl. Phys. 31,2886 (1998). 30. M. Tabuchi, K. Ado, H. Kobayashi, I. Matsubara, H. Kageyama,M. Wakita, S. Tsutsui, S. Nasu, Y. Takeda, C. Masquelier, A. Hirano,and R. Kanno, J. Solid State Chem 141, 554(1998). ( 31. F . J. Berry, C. Greaves, J . G. McManus, M . M o rtimer, and G. Oate s , J. Solid State C hem. 1 30,2 7 2 ( 1 997). ) ( 32. F. J. Berry, J . F . Marco, S. J . Stewart, and H. M. Widatallah, Solid State Commun. 1 1 7, 235 (2001). ) ( 33. G. R. Karagedov, E. A. Konovalova, V . V . B oldyrev, and N . Z. Lyachov, Solid State Ionics 42, 147 (1 9 90). ) 实验方法:Li2CO3和α-Fe2O3的混合物以摩尔比为1:2.5先前尝试采用机械球磨法制备。一半混合物(30g)在空气气氛中煅烧20小时温度在300摄氏度到1000摄氏度之间。另一半混合物在Retsch PM400中干法研磨,使用不锈钢研磨罐(250毫升)和不锈钢球(20毫米)以200转/分运行130小时。球料比(重量比)为1:20。研磨后的粉末随后被以同样方法煅烧。在850摄氏度和1000摄氏度下生产的材料在800摄氏度以下空气中淬火,在炉内缓慢冷却。
关闭-
1/7
-
2/7
还剩5页未读,是否继续阅读?
继续免费阅读全文产品配置单
弗尔德(上海)仪器设备有限公司为您提供《锂铁氧体中机械研磨和煅烧检测方案(研磨机)》,该方案主要用于铁中其他检测,参考标准《暂无》,《锂铁氧体中机械研磨和煅烧检测方案(研磨机)》用到的仪器有德国莱驰行星式球磨仪/机Retsch PM400、德国莱驰高能球磨仪Retsch Emax、卡博莱特盖罗灰化炉CarboliteGeroAAF。
我要纠错
推荐专场
研磨机、研磨仪、粉碎机、球磨机
更多相关方案











 咨询
咨询
