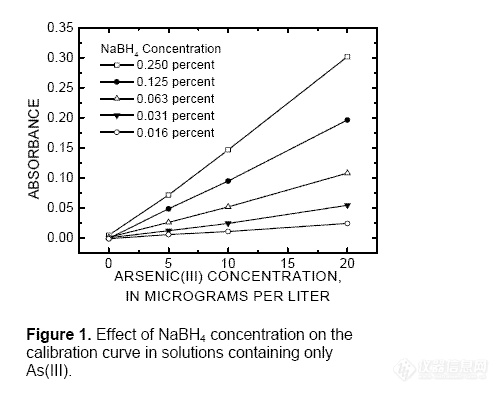
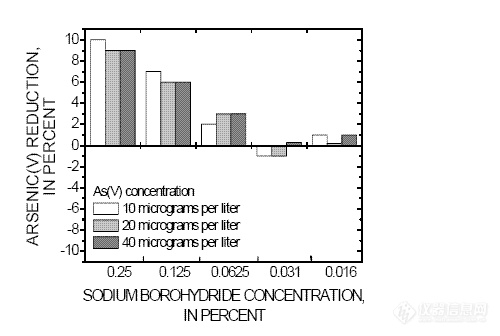
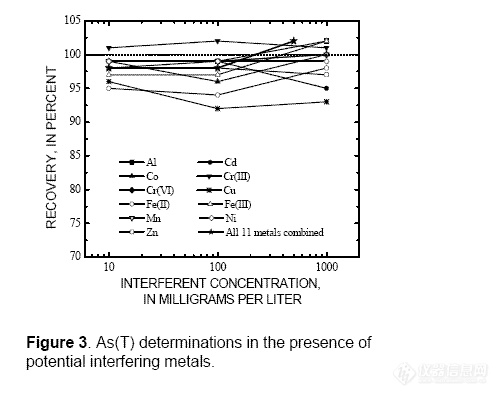
-
+关注
私聊
-

第15楼2005/04/12
Potential Metal Interferences on the
Determination of Arsenic(III)
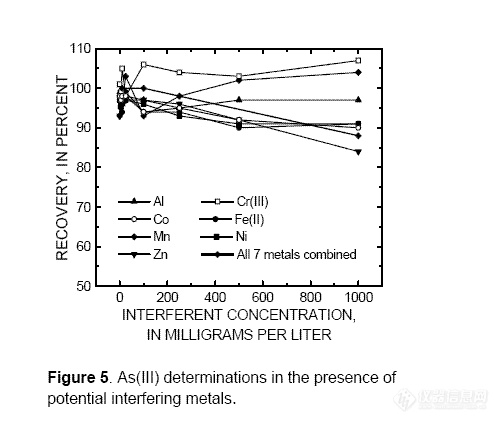
The interference of 16 metal species on
the determination of As(III) was tested by
spiking synthetic samples containing 10 μg/L
As(III) and 10 μg/L As(V) with 0.0001 to
1,000 mg/L Al, Cd, Co, Cr(III), Cr(VI),
Cu(II), Fe(III), Fe(II), Mn, Ni, Pb, Sb(III),
Sb(V), Se(IV), Se(VI), and Zn and
determining the As(III) concentration using
the HGAAS procedure (without cationexchange
treatment). Arsenic(III) recoveries
for samples spiked with Al, Co, Cr(III),
Fe(II), Mn, Ni, and Zn ranged from 84 to 107
percent over the entire concentration range
tested (fig. 5).
Low As(III) recoveries occurred when
Cd exceeded 25 mg/L, Cr(VI) exceeded 0.03
mg/L, Cu(II) exceeded 2.0 mg/L, or Fe(III)
exceeded 1.0 mg/L. Cadmium and Cu(II) are
likely reduced by NaBH4 in the FIAS reaction
chamber and, consequently, arsine production
is limited by the available NaBH4.
Chromium(VI) and Fe(III) inhibit arsine
production by either competing with As(III)
for the available NaBH4 or oxidizing As(III)
to As(V). Arsenic(III) is quickly oxidized as
Fe(III) undergoes photoreduction (Emett and
Khoe, 2001). These synthetic samples were
prepared in clear volumetric flasks and
exposed to light. Therefore, the low As(III)
recovery in the presence of only Fe(III) may
be due to photochemical reactions with
Fe(III) prior to the As(III) determination.
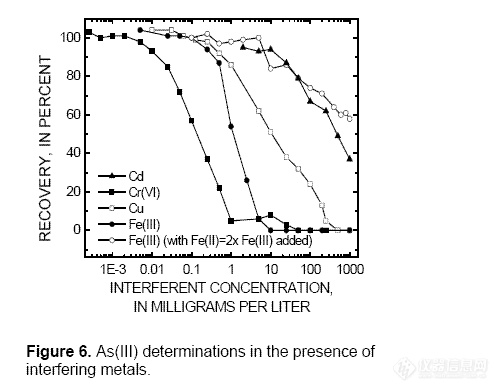
However, when Fe(II) concentrations were
twice the concentration of Fe(III), low As(III)
recoveries occurred only when Fe(III)
exceeded 10 mg/L (fig. 6). Iron(II) relieves
the interference from Fe(III), within a limited
Fe(III) concentration range, by reacting with
free radicals generated as Fe(III) undergoes
photoreduction (Emett and Khoe, 2001).
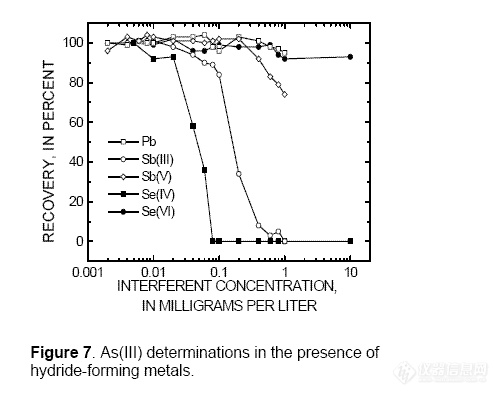
Among the hydride-forming metals, Sb(III) at
concentrations greater than 0.1 mg/L, Sb(V)
greater than 0.4 mg/L, and Se(IV) greater than
0.02 mg/L interfered with As(III)
determinations, whereas Pb and Se(VI) did
not (fig. 7). Antimony(III), Sb(V), and Se(IV)
suppress AsH3(g) production by reacting with
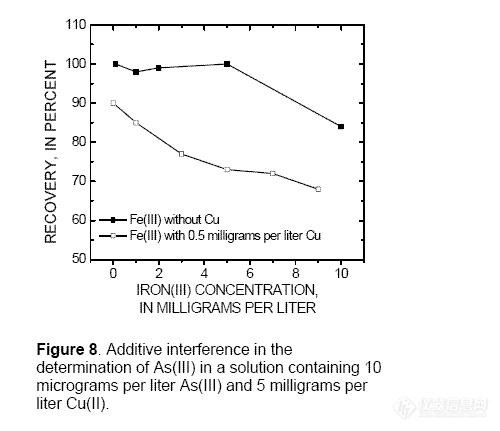
the available NaBH4. An additive effect of
interfering metals on the determination of
As(III) was demonstrated by adding
increasing amounts of Fe(III) to a solution
containing 0.5 mg/L Cu and 10 μg/L As(III)
(fig. 8). Low As(III) recoveries occurred
when the molar ratios of metals to As(III)
were: Cu(II) greater than 120, Fe(III) greater
than 70, Cr(VI) greater than 2, Cd greater
than 800, Sb(III) greater than 3, Sb(V) greater
than 12, or Se(IV) greater than 1. The sample
could not be diluted to an As(III)
concentration (As(III) less than 20 μg/L)
below which these interferences were absent.
Copper(II) and Fe(III) are of primary concern
because water generated from acid mine
drainage potentially contains high
concentrations of Cu(II) and Fe(III).
-
+关注
私聊
-

第20楼2005/04/12
Cation Exchange Separation of
Iron(III) and Copper
Interfering metals can be removed using
cation-exchange resin prior to the
determination of As(III). The relative
selectivity for AG 50W-X8 cation-exchange
resin is 2.95 for Cd, 2.9 for Cu(II), and 1.0 for
H+ (Bio-Rad, 2003). Therefore, as long as the
capacity of the resin (1.7 millieqivalents
(meq) per mL of resin) is not exceeded, Cd
and Cu(II) are expected to be removed from
solution. The relative selectivity for Fe(III) is
not published in the AG 50W instruction
manual.
Laboratory experiments done for this
report demonstrated that AG 50W-X8 cationexchange
resin in the H+ form removed large
amounts of Cu(II) and Fe(III) while
maintaining the existing As(III)/As(T) ratio
(table 1). When the As(III) concentration is
high relative to that of the interfering metal,
the sample may be diluted before analysis to a
Cd, Cu(II), Cr(VI), Fe(III), Sb(III), or Se(IV)
concentration that does not interfere with the
As(III) determination. Chromium(VI) and the
Se and Sb oxyanions will not be removed by
cation exchange.