方案详情文
智能文字提取功能测试中
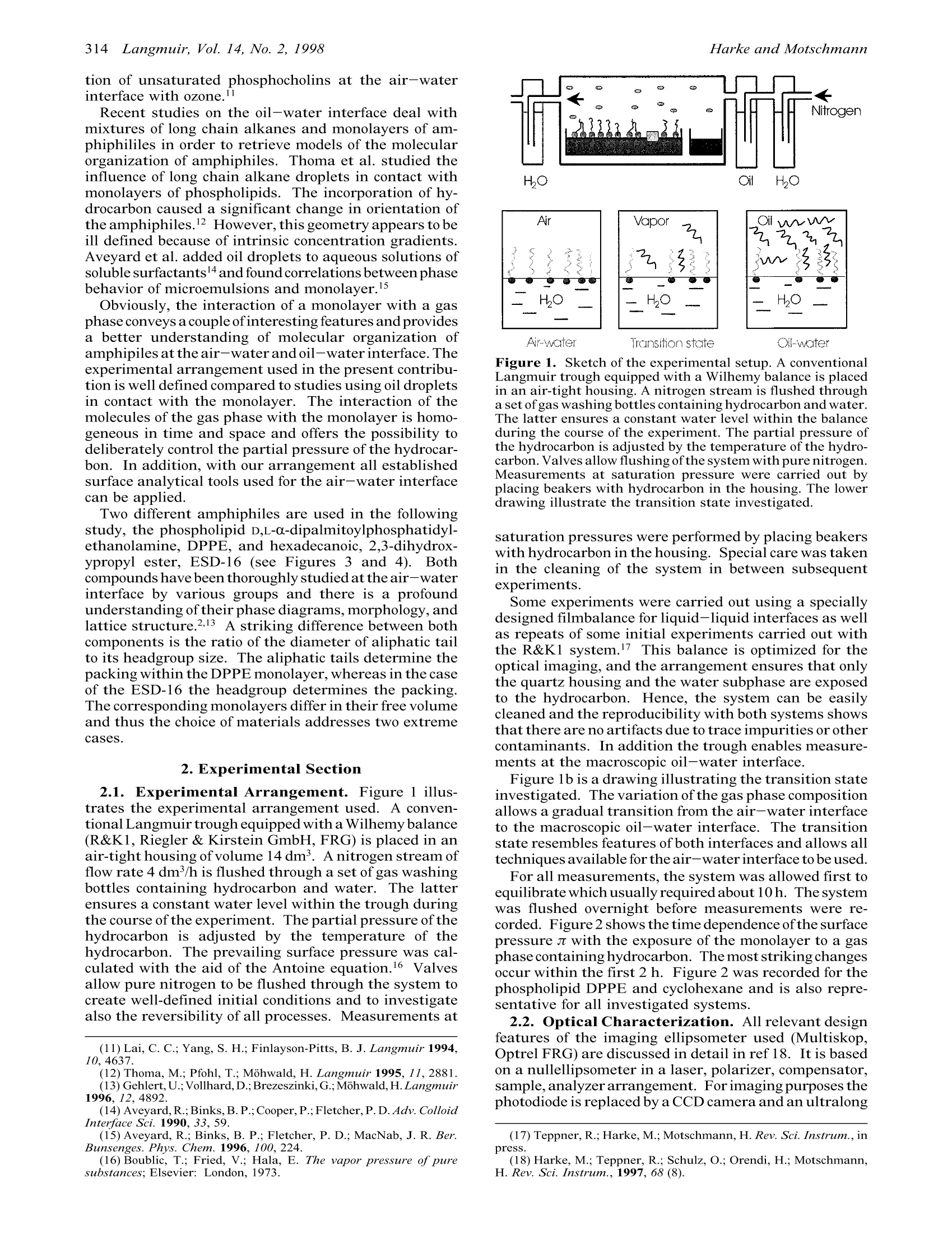
313Langmuir 1998,14,313-318 314Harke and MotschmannLangmuir, Vol. 14, No. 2, 1998 S0743-7463(97)00938-4 CCC: $15.00 C 1998 American Chemical SocietyPublished on Web 01/20/1998 On the Transition State between the Oil-Water andAir-Water Interface M. Harke and H. Motschmann* Max-Planck Institute ofColloids and Interfaces, Rudower Chaussee 5,D-12489 Berlin, Germany Received August 20, 1997. In Final Form: October 21, 1997 Insoluble monolayers at the air-water interface are exposed to a gas phase containing organichydrocarbons. The hydrocarbons are partly incorporated within the monolayer which leads to changesin orientational order and the formation of new phases of different morphology. The transition stateresembles features of the air-water and oil-water interface and the control of the hydrocarbon partialpressure allows continous tuning between both interfaces. The phospholipid D,L-a-dipalmitoylphosphati-dylethanolamine,DPPE,and an esterdiol hexadecanoic acid, 2,3-dihydroxypropylester, ESD-16, are usedas amphiphiles, and pentane, n-hexane, cyclohexane, 2,2-dimethylbutane, n-heptane, n-decane, andn-dodecane are used as hydrocarbons. Both amphiphiles differ in their headgroup size. In DPPE thealiphatic tail determines the packing within the monolayer, but in the case ofESD-16 it is the headgroups.The structural changes are monitiored by surface pressure-area(zt,A)isotherms andimaging ellipsometry.The influence of the chemical nature of the hydrocarbon and the effect of the partial pressure of thehydrocarbon on the monolayer structure are assessed. 1. Introduction The oil-water interface plays a decisive role in manyaspects of daily life. Despite its importance, still little isknown about the organization of amphiphiles at thisinterface and their corresponding static and dynamicinterfacial properties. This is mainly due to severerestrictions on the analytical tools imposed by this buriedinterface. Many powerful techniques suitable for inves-tigating the liquid-air interface such as X-ray reflecto-metry or X-ray diffraction cannot be applied at liquid-liquid interfaces.X-ray diffraction in particular hasprovided a new insight into the organization of insolublemonolayers at the air-water interface.2 These measure-ments allow determination of the lattice structure withindifferent phases and provide corroborative evidence forthe fairly complex phase diagrams as retrievedby surfacetension measurements.3Unfortunately this powerfultechnique is not available for investigation of the mac-roscopic oil-water interface. The present paper focuses on a transition state betweenthe macroscopic oil-water and the air-water interfaces.The common theme of the following set of experiments is.as follows: A monolayer of a water insoluble amphiphileis exposed to a gas phase containing organic hydrocarbonsat a well-defined partial pressure. The hydrocarboninteracts with the monolayer and is incorporated,leadingto changes in the orientational order, lattice structure,and morphology. As a result, a transition state evolves,which resembles features of both the bare air-water aswell as the macroscopic oil-water interface. The controlof the partial pressure ofthe hydrocarbon allows a detailedassessment of structural changes from the air-water tothe macroscopic oil-water interface. The present studyfocuses on this intermediate state and utilizes mainlysurface tension measurements for the determination ofn,4-isotherms and imaging ellipsometry for the visualiza- ( * To whom c o rrespondence should be addressed: electronicmail, motschma@mpikg.fta-berlin.de. ) ( (1)Mohwald, H. Rep. Prog. Phys. 1993, 56, 653. ) ( (2) Mohwald, H. Annu. R e v. P h ys. C h em. 19 9 0, 41, 441. ) tion ofthe morphology on an mesoscopic length scale. Thistransition state can also be investigated by all analyticaltools available for the bare air-water interface. X-raydiffraction data on this system will be discussed in a futurecontribution of ours. The manipulation of organic monolayers via the gasphase has been widely ignored compared to the numerousstudies dealing with the influence of the subphase on amonolayer.4.5 The subphase properties have been changedby dissolving salts, by pH changes, or by adding solublesurfactants. Previous studies on the influence of the gasphase on monolayers included investigations of theinfluence of argon, xenon, and nitrogen. However, theseinert gases had no impact on the monolayer. Theadsorption of hydrocarbons on the bare water interfacewas investigated by Baumer et al., and the decrease inthe surface tension provided evidence of the adsorptionof hydrocarbons at the bare water surface. This arrange-ment is now used for fundamental wetting studies in orderto retrieve the nature of the underlying forces drivingwetting phenomena. A monolayer of an insoluble amphiphile at the air-water provides additional adsorption sites for hydrocar-bons from the gas phase. This process was first studiedby Dean et al. using surface tension measurements, andon the basis ofaphenomenological thermodynamic model,the number of molecules incorporated in the monolayerwas estimated. Clements et al. and Ueda et a1.10 used the samearrangement to study aspects ofthe interaction of volatilenarcotics on the cell membrane using Langmuir films assimple models for the latter. Also chemical reactions ofLangmuir monolayer with molecules from the gas phasehave been investigated, e.g., Lai et al. studied the reac- ( (4) M cConnell, H . M. Annu. Rev. P hys. Chem. 1991, 4 2, 171 . ) ( (5) Gaines,G. L. Insoluble Monolayer at liquid gas interfaces; W iley:New York, 1 996. ) ( (6) Baumer,D. F i ndenegg,G.J.Colloid. Interface Sci. 1981,85,118. ) ( (7) Pfohl, T .; Mohwald, H.; Riegler,H. Langmuir, in p ress. ) ( (8) D ean, R. B . Kolloid Z. 1953, 123, 1 17. ) ( (9) C l ements, J. A.; Wilson, K. M. Physiology 1965,48 , 10 0 9. ) ( (10) Ueda,J.; Shieh, D. D.; E yring, H. Anesthesiolgy 1 9 74, 41,217. ) tion of unsaturated phosphocholins at the air-waterinterface with ozone.l Recent studies on the oil-water interface deal withmixtures of long chain alkanes and monolayers of am-phiphililes in order to retrieve models of the molecularorganization of amphiphiles. Thoma et al. studied theinfluence of long chain alkane droplets in contact withmonolayers of phospholipids. The incorporation of hy-drocarbon caused a significant change in orientation ofthe amphiphiles.12 However, this geometry appears to beill defined because of intrinsic concentration gradients.Aveyard et al. added oil droplets to aqueous solutions ofsoluble surfactants4and found correlations between phasebehavior of microemulsions and monolayer. Obviously, the interaction of a monolayer with a gasphase conveys a couple ofinteresting features andprovidesa better understanding of molecular organization ofamphipiles at the air-water and oil-waterinterface.Theexperimental arrangement used in the present contribu-tion is well defined compared to studies using oil dropletsin contact with the monolayer. The interaction of themolecules of the gas phase with the monolayer is homo-geneous in time and space and offers the possibility todeliberately control the partial pressure of the hydrocar-bon. In addition, with our arrangemmenit nall establishedsurface analytical tools used for the air-water interfacecan be applied. Two different amphiphiles are used in the followingstudy, the phospholipid D,L-a-dipalmitoylphosphatidyl-ethanolamine, DPPE, and hexadecanoic, 2,3-dihydrox-ypropyl ester, ESD-16 (see Figures 3 and 4). Bothcompounds have been thoroughly studied at the air-waterinterface by various groups and there is a profoundunderstanding of their phase diagrams, morphology,andlattice structure.2,13 A striking difference between bothcomponents is the ratio of the diameter of aliphatic tailto its headgroup size. The aliphatic tails determine thepacking within the DPPE monolayer, whereas in the caseof the ESD-16 the headgroup determines the packing.The corresponding monolayers differ in their free volumeand thus the choice of materials addresses two extremecases. 2. Experimental Section 2.1. Experimental Arrangement. Figure 1 illus-trates the experimental arrangement used. A conven-tional Langmuir trough equipped with a Wilhemy balance(R&K1, Riegler & Kirstein GmbH, FRG) is placed in anair-tight housing of volume 14 dm3. A nitrogen stream offlow rate 4 dm/h is flushed through a set of gas washingbottles containing hydrocarbon and water. The latterensures a constant water level within the trough duringthe course of the experiment. The partial pressure of thehydrocarbon is adjusted by the temperature of thehydrocarbon. The prevailing surface pressure was cal-culated with the aid of the Antoine equation.16 \Valvesallow pure nitrogen to be flushed through the system tocreate well-defined initial conditions and to investigatealso the reversibility of all processes. Measurements at ( (11) Lai, C. C.; Yang, S. H. ; Finlayson-Pitts , B. J. Langmuir 1994, 10, 4637. ) ( (12) Thoma, M.; P fohl, T.; Mohwald, H. Langmuir 1995, 11, 2881.(13) Gehlert, U.;Vollhard,D.; Brezeszinki,G.;Mohwald,H . Langmuir 1996, 1 2,4892. ) ( (14) Aveyard,R.;Binks, B. P.; Cooper,P.;Fletcher, P. D. Adv. ColloidInterface Sci. 1990, 3 3, 5 9. ) ( (15) Aveyard, R.; B inks, B . P.; F letcher, P . D .; MacNab, J. R . B e r.Bunsenges. Phys. Chem. 1996,100,224. ) ( (16) Boublic, T.; Fried, V.; Hala, E. The v apor pressure o f pure substances; Elsevier: London, 1973. ) Air-water ransition state Oil-water Figure 1. Sketch of the experimental setup. A conventionalLangmuir trough equipped with a Wilhemy balance is placedin an air-tight housing. A nitrogen stream is flushed througha set of gas washing bottles containing hydrocarbon andwater.The latter ensures a constant water level within the balanceduring the course of the experiment. The partial pressure ofthe hydrocarbon is adjusted by the temperature of the hydro-carbon. Valves allow flushing ofthe system with pure nitrogen.Measurements at saturation pressure were carried out byplacing beakers with hydrocarbon in the housing. The lowerdrawing illustrate the transition state investigated. saturation pressures were performed by placing beakerswith hydrocarbon in the housing. Special care was takenin the cleaning of the system in between subsequentexperiments. Some experiments were carried out using a speciallydesigned filmbalance for liquid-liquid interfaces as wellas repeats of some initial experiments carried out withthe R&K1 system. This balance is optimized for theoptical imaging, and the arrangement ensures that onlythe quartz housing and the water subphase are exposedto the hydrocarbon. Hence, the system can be easilycleaned and the reproducibility with both systems showsthat there are no artifacts due to trace impurities or othercontaminants. In addition the trough enables measure-ments at the macroscopic oil-water interface. Figure 1b is a drawing illustrating the transition stateinvestigated. The variation of the gas phase compositionallows a gradual transition from the air-water interfaceto the macroscopic oil-water interface. The transitionstate resembles features of both interfaces and allows alltechniques available for the air-water interface to be used. For all measurements, the system was allowed first toequilibrate which usually requiredabout 10h. The systemwas flushed overnight before measurements were re-corded. Figure 2 shows the time dependence ofthe surfacepressure z with the exposure of the monolayer to a gasphase containing hydrocarbon. The moststriking changesoccur within the first 2 h. Figure 2 was recorded for thephospholipid DPPE and cyclohexane and is also repre-sentative for all investigated systems. 2.2. Optical Characterization. All relevant designfeatures of the imaging ellipsometer used (Multiskop,Optrel FRG) are discussed in detail in ref 18. It is basedon a nullellipsometer in a laser, polarizer, compensator,sample, analyzer arrangement. Forimaging purposes thephotodiode is replaced by a CCD camera and an ultralong ( (17) Teppner, R.; Harke, M.; Motschmann,H. Rev. Sci. Instrum., inpress. ) ( (18) Harke, M.; Teppner, R .; Schulz, O.; Orendi, H. ; Motschmann, H. R ev. S ci. Instrum., 1 997, 68(8). ) Time [S] Figure 2. Surface pressure of DPPE as a function of time t.At t =0 the DPPE monolayer was exposed to a gas phasecontaining n-hexane. Similar features were observed for allhydrocarbons used. Figure 3. Chemical structure of the phospholipid D,L-a-dipalmitoylphosphatidylethanolamin,DPPE. The aliphatic taildetermines the packing within the monolayer. microscope objective with a working distance of 34 mmand a numerical aperture (NA) = 0.22 is inserted. Aalgorithm used for data analysis is described in ref 19. 2.3. Materials. The chemical structure of the phos-pholipid D,L-a-dipalmitoylphosphatidylethanolamine(DPPE) is presented in Figure 3. DPPE was purchasedfrom Sigma-Aldrich and used as received. The chemical structure of the hexadecanoic acid, 2,3-dihydroxypropylester(ESD-16), is presented in Figure 4.ESD-16 was purchased from Sigma-Aldrich and used asreceived. All hydrocarbons were purchased from Sigma-Aldrich.1giTrace impurities are always a major concern in theinvestigation of interfacial properties. Some trace im- ( (19) Harke, M.; Stelzle, M.; Motschmann, H. Thin Solid Films 1996, 284,41 2 . ) ( (20) Goebel, A.; Lunkenheimer, K. Langmuir 19 9 7, 13 , 369. ) Figure 4. Chemical structure of the hexadecanoic acid,2,3-dihydroxypropyl ester,ESD-16. The bulky headgroup deter-mines the packing within the monolayer. Area per molecule [A] Figure 5. n,A-isotherm of the phospholipid DPPE at the air-water interface and in contact with a gas phase containingn-hexane,cyclohexane, and 2,2-dimethylbutane. The presenceofthe hydrocarbon in the gas phase induces a coexistence region.All isotherms were recorded at 22°C and at saturation vaporpressure of the hydrocarbon. purities possess a high affinity for the interfaceofinterest,and thus the interface becomes enriched. These impuritiesare efficiently removed by the shaking ofthe hydrocarbonwith water and subsequent decantation of the organicphase. This purification method is analyzed in ref 20. 3. Results and Discussion 3.1.u,4-Isotherm. 3.1.1. DPPE: The n,A-isothermof DPPE recorded at 20 °C at the air-water interface isfairly simple and presented in Figure 5. During thecompression of the monolayer a homogeneous liquid-condensed phase is formed at a molecular area of 52 A2.Significant changes in the n,A-isotherm occur once thesystem is exposed to a gas phase containing hydrocarbon.Figure 5 presents the corresponding isotherms for cyclo-hexane, n-hexane, and 2,2-dimethylbutane. The mostprominent feature is a newly formed plateau in the n,A-isotherm. The area per molecule at which the onset ofthe plateau occurs is fairly independent of the specific Figure 6. Influence of the n-heptane partial pressure on thecorresponding isotherm of ESD-16. The prevailing partialpressure is normalized by its saturation pressure as indicatedby the numbers in the graph. isomer. Only a slight shift to higher areas per moleculeis observed for 2,2-dimethylbutane, which might beattributed to its size. Furthermore all isotherms areshifted to higher surface pressures, , and a correlationwith the saturation vapor pressure,psat, ofthe hydrocarboncan be found; the psat values of the different hydrocarbonsare 35.1 kPa cylohexane, 16.2 kPa n-hexane, and 10.3kPa 2,2-dimethylbutane.16 The partial pressure deter-mines the number of molecules which strike the surfaceper unit area and time. The liquid-condensed region isformed at a given molecular area which is independentofthe presence and specific chemical nature ofthe hexane1somer. To summarize, the presence a ofhydrocarbon in the gasphase induces the formation of new phases. The specificchemical nature of the hydrocarbon has only a minorimpact on the features of the st,4-isotherm. The hydro-carbon is not incorporated in the liquid condensed phaseof DPPE. 3.1.2. ESD-16. The influence of the partial pressureon the j,4-isotherm of ESD-16 was investigated usingn-heptane. Figure 6 shows the corresponding isothermsrecorded at 22℃ at different partial pressures ofn-heptane. The prevailing partial pressure of n-heptaneis normalized by its saturation vapor pressure as indicatedin the inset of Figure 6. The first onset in the surfacepressure at the air-waterinterface occurs at a moleculararea of about 70 A2. At a molecular area of 52 A2, acoexistence region between a liquid condensed and liquidexpanded phase is observed. The low compressibility ofthe liquid condensed phase causes a steep increase in aat low areas per molecule. The presence ofn-heptane in the gas phase does inducechanges in the n,A-isotherm. As depicted in Figure 6, theonset of the surface pressure occurs at higher areas permolecule. There is still a coexistence region but it is shiftedto higher surface pressures, Jc, and lower areas permolecule. The plateau value ofthe surface pressure, Jtc,does not depend linearly on the partial pressure of thehydrocarbon. The liquid condensed phase is formed at amolecular area of 22 A2 and is independent of theprevailing n-heptane partial pressure. Hence, no hydro-carbon is incorporated in the liquid condensed phase. Figure 7. (a) Morphology of DPPE in the low-pressure regionof the isotherm. There are two distinct phases in the low-pressure region of the isotherm. The very same features wereobserved within the homologous series ofn-alkanes. The imagewas recorded at = 10 mN/m in a gas phase containingcyclohexane at its saturation vapor pressure and at a temper-ature of 22°C. Microdoplets of hydrocarbon are formed on thedomains. (b) The domains which are observerd in the low-pressure region dissolve in the plateau of the n,A-isotherm.The growth of the new phase occurs in the center of the domainas well as at the edge. 3.22.. Imaging Ellipsometry. 3.2.1. DPPE:: Theprocess of spreading usually leads to nonequilibriumstructures of floating islands at the air-water interface.The shape of these nonequilibrium structures dependsstrongly upon the preparation process such as spreadingagent and concentration of the solution etc. However,with the first onset in the surface pressure, j, a homog-enous phase of a liquid expanded monolayer is formed. Inthis region of the isotherm, ellipsometric images reveala homogeneous phase with uniform reflectivity. With the introduction of hydrocarbon in the gas phase,completely different features are observed. The onset inthe surface pressure, n, does not lead to the formation ofauniformphase. Instead, imaging ellipsometry identifiestwo well-definedphases in the low-pressure region. Figure7shows the morphologyofDPPE recorded in acyclohexaneatmosphere at a saturation pressure Jsat. Domains of acircular shape with uniform optical properties are embed-ded in a homogeneous matrix. Microdroplets form on thedomains. Presumedly, the microdroplets consist of ahydrocarbon which does not wet the underlying monolayer. Further compression leads to the formation of a newphase in the plateau of the ,4-isotherm. The growth ofa new phase occurs not only at the edge but also withinthe center of the domain as shown in Figure 7. A blackmatrix of a less densely packed monolayer separates thenew from the old phase.The new phase consists ofdomains of circular shape. They vary in size of between10 and 20 um and are significantly smaller than theircounterparts at the air-water interface. This and theloss of contrast due to the presence of hydrocarbon in thegas phase makes it also more challenging for imaging Figure 8. (a) Morphology of DPPE in the plateau of theisotherm under various hydrocarbons. Domains with circularshape and an internal order are observed. The molecule adopta common azimuthal angle within each segment ofthe domain.The image was recorded for cyclohexane but it is also repre-sentative for other hydrocarbons: pentane, n-hexane, n-heptane, n-decane. (b) Within the homologous series the firstchange in morphology occurs with dodecane. In this case thedomain lack an internal structure and the amphiphiles areorganized with an upright orientation. under an oblique angle of incidence. In addition, an1minternal structure with 6-fold symmetry is observed.Themolecules are arranged in each segment with a commonazimuthal angle. Hence the presence ofhydrocarbonleadsto the formation of a highly ordered crystalline state.Figure 8 shows a representative plot. The observedmorphology is identical for the following hydrocarbons:cyclohexane,pentane,hexane,n-heptane,n-decane. Thefirst deviations within the homologous series were ob-served with dodecane. Here the domains lack any internalstructure as presented in Figure 8b. The molecules areorientated upright or an azimuthal isotropic moleculararrangement is adopted. 3.2.2. ESD-16. At the air-water interface, ESD-16forms domains of a circular shape as presented in Figure9a. A remarkable feature is an internal structure with7-fold symmetry. In the majority of cases a commonintersection point is located in the center of the domain,but in some instances the intersection point is observedat the edge of the domain. Thelatter is ofa cardioid shapeand quite frequently possesses less than seven segments.The structure and morphology were investigated in detailin ref 13. In each segment the molecules possess anidentical azimuthal angle d and there is a discontinuityind between adjacent segments. Under n-heptane atsaturation pressure, the morphology of ESD-16 displayscompletely different features as illustrated in Figure 9c.At a hydrocarbon partial pressure close to saturation, thedomains adopt a dendritic shape andlack any internalstructure. The dendritic shape of the domains does notchange during the course of the experiment (24h), whichmight be explained in terms of a significant decrease in a Figure 9. (a) Ellipsometric image of domains of ESD-16recorded at the air-water interface.The domains are ofcircularshape and possess a 7-fold internal symmetry. Each segmentpossesses a uniform reflectivity and in the majority of casesthere is a common intersection point in the center ofthe domain.(b) Morphology of the domains in the coexistence region ofESD-16 recorded at 22 °C and a partial pressure of heptan p=psat.(c) Morphology ofESD-16 recorded in contact with a gas phasecontainingn-heptane at its saturation vapor pressure.Dendriticdomains with no internal structure are observed. The observedfeatures do not change within 24 h. line tension. Partial pressures below saturation, Psat,preserve the internal structure of the domains.5.Arepresentative image is shown in Figure 9b, which wasrecorded at a partial pressure of 0.6 psat. All domainshave lost their circular shape. In addition, a dip at onesegment is observed which in some cases joins the insectionpoint ofall segment boundaries at the center ofthe domain. Nonequilibrium structures under a gas phase containinghydrocarbon produced under a fast compression interest- Figure 10. Nonequilibrium of ESD-16 produced by a fastmonolayer compression in contact with n-heptane vapors.Contrary to the air-water interface, each arm consists of tworegions of different reflectivity. The growth of the domain is inthe direction of the boundary of two segments. ing features compared to their counterparts at the air-water interface. Nonequilibrium structures at the air-water interface are of dendritic shape. Each finger possessits own unique reflectivity different from adjacent seg-ments. The growth ofeach domain is directed to the half-angle of a segment.21 UUnder hydrocarbon vapors eacharm consists of two regions of a different reflectivity asshown in Figure 10. Hence, in this case the growth of thedomain is governed by the direction of the boundary oftwo segments. 4. Summary and Conclusions The influence of a gas phase containing various hy-drocarbons at well-defined partial pressures on insolublemonolayers of DPPE and ESD-16 was investigated bysurface tension measurements and imaging ellipsometry.The latter provides information on a mesoscopic lengthscale. Different isomers of hexane demonstrate that the ( (21) Gehlert, U.; Weidemann, G.; V o llhardt, D . J. Colloid Interface Sci.1995,174,392. ) specific shape of the molecules has only a minor impacton thes,4-isotherm as well as on the observed morphology. In the case of DPPE, the presence of a hydrocarboninduces a coexistence region in its ,4-isotherm. In theplateau region domains ofcircular shape with an internal6-fold symmetry are observed. The morphology is in thiscase independent of the hydrocarbons present (evenaromatic ones). Within each segment of the domains, themolecules possess a common azimuthal angle . The liquid condensed phase is formed at a givenmolecular area independent of the partial pressure andthe specific hydrocarbon used. Hence the hydrocarbon isnot incorporated within the liquid condensed phase.During compression it is squeezed out of the monolayerand forms droplets on it. Two phases were observed withinthe low-pressure region. The tilt angle of the monolayerdepends on the presence ofhydrocarbon and is specific tothe shape of both amphiphile and hydrocarbon. The coexistence region observed with ESD-16 at theair-water interface is preserved in contact with hydro-carbon. However,the onset ofthe plateau is shifted towardlower molecular areas with increasing hydrocarbon partialpressure. At the air-water interface,domains with sevensegments of uniform reflectivity are observed. At thesaturation vapor pressure ofn-heptane, dendritic domainsare formed and the inner structure of the domains is lost.The hydrocarbon is incorporated which leads to an uprightorganization of amphiphiles. There is a continuoustransition between both states with increasing partialpressure. At a partial pressure below saturation, thecircular shape of the domains becomes deformed and thecommon intersection point of all the segment is quitefrequently shifted to the edge of the domain. Results on the interaction of monolayers with moleculesfrom a gas phase contribute to the understanding of themolecular organization of amphiphiles at the air-waterand oil-waterinterface. With our arrangement it is possi-ble to use all established analytical tools suitable for theinvestigation of the air-water interface. The conditionsare well defined with a uniform interaction in time andspace, and the partial pressure of the hydrocarbon can becontrolled. Acknowledgment. The authors thank Professor H.Mohwald for encouraging discussions and his support andalso Dr. Martina Bree for helpful discussions.
关闭-
1/6

-
2/6
还剩4页未读,是否继续阅读?
继续免费阅读全文产品配置单
北京欧兰科技发展有限公司为您提供《油水空气中关于油/水, 空气/水界面的过渡态研究检测方案(椭偏仪)》,该方案主要用于其他中关于油/水, 空气/水界面的过渡态研究检测,参考标准《暂无》,《油水空气中关于油/水, 空气/水界面的过渡态研究检测方案(椭偏仪)》用到的仪器有组合式多功能椭偏仪、Imager sCMOS PIV相机、德国LaVision PIV/PLIF粒子成像测速场仪。
我要纠错
推荐专场
CCD相机/影像CCD
更多相关方案













 咨询
咨询