方案详情文
智能文字提取功能测试中
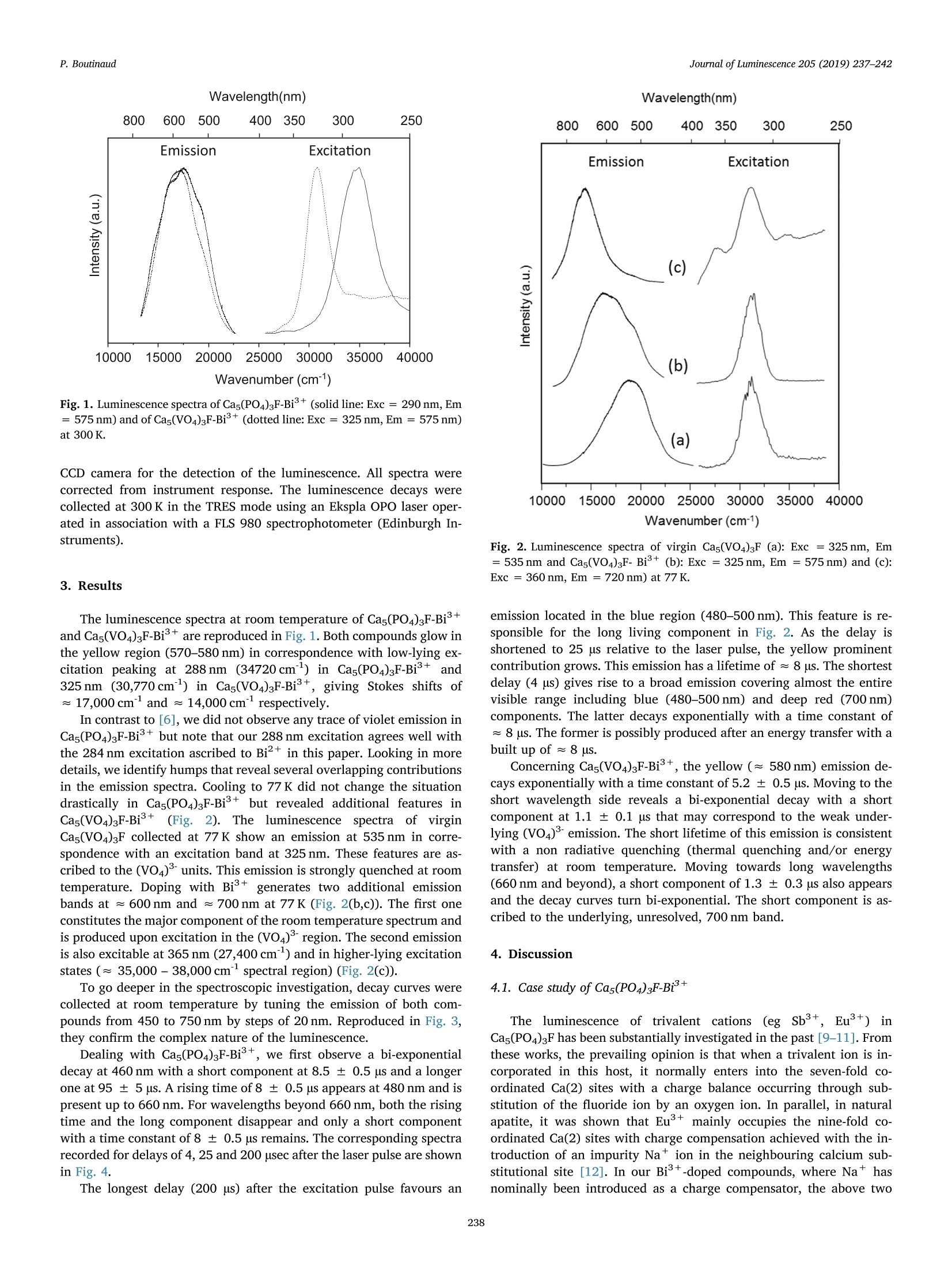
Journal of Luminescence 205 (2019) 237-242Contents lists available at ScienceDirectJournal of Luminescence P. BoutinaudJournal of Luminescence 205 (2019) 237-242Wavelength(nm) journal homepage: www.elsevier.com/locate/jlumin Spectroscopic investigations of calcium fluoroapatites doped with Bi Philippe Boutinaud Clermont Universite Auvergne, SIGMA Clermont, Institut de Chimie de Clermont-Ferrand, BP 10448, 63000 Clermont-Ferrand, France A RTICLEINFO ABSTRA C T Keywords:Bi3+Charge transferLuminescencePairs The spectral and dynamical properties of Biin the calcium fluorophosphates Cas(PO4)3F and Cas(VO4)3F areinvestigated at room and liquid nitrogen temperature. The data are interpreted at the light of recent semi-empirical models that allow calculating the energy of A-like (isolated Bi), D-like (metal-metal charge transfer)and pair-like transitions from the knowledge of the crystal structure of the host lattices. It is concluded that theattribution of the luminescence features to isolated Bi+ions is not always an obvious option and that theluminescence can also be very satisfactorily interpreted in terms of charge transfer processes, either within Bi+-Bi+pairs in Cas(PO4)3F-Bi+or within Bi+-Bi+and Bi +-v5+pairs in the newly reported Cas(VO4)3F-Bi+. 1. Introduction Although investigated for decades, the spectroscopy of trivalentbismuth in solids is still not totally fixed. The reason is that the emissionof this ion can arise from different kinds of excited states: "regular”states belonging to isolated Biions or states with charge transfercharacter, including Bi+pairs. Depending on the doping rate, on thenature of the host lattice and on external stresses like temperature orpressure, more than one emission process can occur [1-4].This con-comitancy usually generates complex spectra and is the origin of manycontroversies in terms of spectral assignments. As a case study, theluminescence properties of(lBi3+intheecalciumffiluoro-apatiteCas(PO4)3F are variously interpreted in the archival literature: Ac-cording to Zhu et al. [5], Cas(PO4)3F-Bi+ shows a greenish-yellowemission at 538 nm in correspondence with an excitation band at273 nm, whereas according to Li et al. [6], Cas(PO4)3F-Bi+ glows inthe violet (397nm) and green (520nm) spectral regions upon excita-tion at 354 nm and 284nm, respectively. In [5], the excitation band hasbeen ascribed to the regular so (6s)-3P1 (6s'6pl) transition (so-called A transition) of Bi+ and the resulting emission, Stokes shifted byan amount as large as 18,000 cm, is declared to belong to isolatedBi+ ions. In [6], the emission having the smallest Stokes shift(3250 cm) has been ascribed to regular A transition of isolated Bi+located in the Ca(2) sites of the apatite lattice (i. e. seven-coordinatedsites (6 h) with Cs point symmetry), while the emission with the largestStokes shift (16,000 cm) has been ascribed to Bi+ located in Ca(1)sites (i. e. nine-coordinated sites (4 f) with C3 point symmetry). Such adiscrepancy in addressing the origin of the spectroscopic properties ofBi illustrates well how confusing the situation can be. The motivationof the present paper is to bring some elements of clarification in this connection at the light of recently developed models [1-3]. In thispurpose, the spectroscopic properties of Cas(PO4)3F-Bi+ are re-in-vestigated and newly interpreted. In addition, we report original resultson the calcium fluoro-apatite Cas(VO4)3F-Bi+ where the substitutionof phosphorus to vanadium creates the possibility for a Bi+-to-v5+metal to metal charge transfer (MMCT) state to form as in the case ofYVO4-Bi+[7]. 2. Experimental The synthesis of Cas(PO4)3F-Bi+, Cas(VO4)3F-Bi+ and virgin,i1lCas(VO4)3F was carried out in the solid state by following the indica-tions reported in [8]. In this paper, x mol% NaCOs (Acros Organics,99.6%) was introduced to compensate for the substitution of x mol% oftrivalent dopant to) Ca2+. Thisgives the chemicalfformulaeNaxBixCa1-2x(MO4)3F, withM=P,Vandx=0.01. The other sourcechemicals were Bi203 (Acros Organics, > 99.9%), CaCOs (Strem Che-micals > 99.9%), CaF2 (Acros Organics,> 99%), (NH4)2HPO4 (FlukaAnalytical,>99%), NHVO4 (Acros Organics, 99.5%). The stoichio-metric mixture was placed in an alumina crucible, positioned at thebottom of a 50 cm-long closed silica tube and heated at 700℃ for 15 hunder a confined atmosphere of argon, cooled, crushed and then heatedat 1200°℃ under argon for 4 h. When necessary, this thermal treatmentwas repeated until a single phase was obtained. The X-ray diffractionpatterns confirmed the formation of Cas(PO4)3F in its P63/m hexagonalform (ICSD 193552) and of Cas(VO4)3F in its triclinic P-1 form (ICSD172997). The steady-state luminescence was recorded at 300 and 77 Kusing a setup consisting of a xenon lamp coupled to a Triax 180monochromator (Jobin Yvon Horiba) as excitation source and a Triax550 monochromator (Jobin Yvon Horiba) equipped with a N2 cooled ( E-mail address: p h il i ppe.bo u ti n aud@sig m a- c lermon t .fr . ) ( Received 18 June 2018; Received i n revised form 7 September 2018; Accepted 10 September 2018 Available online 11 September 2018 ) ( 0022-2313/C 2018 Elsevier B.V. All ri g hts reserved. ) Fig. 1. Luminescence spectra of Cas(PO4)3F-Bi+(solid line: Exc= 290 nm, Em=575 nm) and of Cas(VO4)3F-Bi+ (dotted line: Exc = 325 nm, Em =575nm)at 300 K. CCD camera for the detection of the luminescence. All spectra werecorrected from instrument response. The luminescence decays werecollected at 300 K in the TRES mode using an Ekspla OPO laser oper-ated in association with a FLS 980 spectrophotometer (Edinburgh In-struments). 3. Results The luminescence spectra at room temperature of Cas(PO4)3F-Biand Cas(VO4)3F-Bi+ are reproduced in Fig. 1. Both compounds glow inthe yellow region (570-580 nm) in correspondence with low-lying ex-citation peaking at 288 nm (34720cm) in Cas(PO4)3F-Bi+ and325nm (30,770cm) in Cas(VO4)3F-Bi+, giving Stokes shifts of≈17,000cmand ≈ 14,000cmrespectively. In contrast to [6], we did not observe any trace of violet emission inCas(PO4)3F-Bi+ but note that our 288 nm excitation agrees well withthe 284 nm excitation ascribed to Bi+ in this paper. Looking in moredetails, we identify humps that reveal several overlapping contributionsin the emission spectra. Cooling to 77 K did not change the situationdrastically in Cas(PO4)3F-Bi+ but revealed additional features inCas(VO4)3F-Bi+((Fig.2). The luminescencespectra of virginCas(VO4)3F collected at 77 K show an emission at 535 nm in corre-spondence with an excitation band at 325nm. These features are as-cribed to the (VO4) units. This emission is strongly quenched at roomtemperature. Doping with Bi+ generates two additional emissionbands at ≈600 nm and ≈ 700nm at 77 K (Fig. 2(b,c)). The first oneconstitutes the major component of the room temperature spectrum andis produced upon excitation in the (VO4)region. The second emissionis also excitable at 365 nm (27,400 cm) and in higher-lying excitationstates (≈ 35,000-38,000 cmspectral region) (Fig.2(c)). To go deeper in the spectroscopic investigation, decay curves werecollected at room temperature by tuning the emission of both com-pounds from 450 to 750 nm by steps of 20 nm. Reproduced in Fig. 3,they confirm the complex nature of the luminescence. Dealing with Cas(PO4)3F-Bi+, we first observe a bi-exponentialdecay at 460 nm with a short component at 8.5± 0.5 us and a longerone at 95 ± 5 us. A rising time of8 ± 0.5 us appears at 480 nm and ispresent up to 660 nm. For wavelengths beyond 660 nm, both the risingtime and the long component disappear and only a short componentwith a time constant of 8 ± 0.5 us remains. The corresponding spectrarecorded for delays of 4, 25 and 200 usec after the laser pulse are shownin Fig. 4. The longest delay (200 us) after the excitation pulse favours an Fig. 2. Luminescence spectra of virgin Cas(VO4)3F (a): Exc = 325 nm, Em=535 nm and Cas(VO4)3F- Bi+(b): Exc =325 nm, Em =575 nm) and (c):Exc=360 nm, Em =720 nm) at 77 K. emission located in the blue region (480-500nm). This feature is re-sponsible for the long living component in Fig. 2. As the delay isshortened to 25 us relative to the laser pulse, the yellow prominentcontribution grows. This emission has a lifetime of ≈8 us.The shortestdelay (4 us) gives rise to a broad emission covering almost the entirevisible range including blue (480-500nm) and deep red (700 nm)components. The latter decays exponentially with a time constant of=8 us. The former is possibly produced after an energy transfer with abuilt up of ≈8 us. Concerning Cas(VO4)3F-Bi+, the yellow (≈ 580 nm) emission de-cays exponentially with a time constant of 5.2 ± 0.5 us. Moving to theshort wavelength side reveals a bi-exponential decay with a shortcomponent at 1.1 ± 0.1 us that may correspond to the weak under-lying (VO4)emission. The short lifetime of this emission is consistentwith a non radiative quenching (thermal quenching and/or energytransfer) at room temperature. Moving towards long wavelengths(660 nm and beyond), a short component of 1.3 ± 0.3 us also appearsand the decay curves turn bi-exponential. The short component is as-cribed to the underlying, unresolved,700 nm band. 4. Discussion 4.1. Case study of Cas(PO4)3F-Bi+ The luminescence of trivalent cations (eg Sb+, Eu) inCas(PO4)3F has been substantially investigated in the past [9-11]. Fromthese works, the prevailing opinion is that when a trivalent ion is in-corporated in this host, it normally enters into the seven-fold co-ordinated Ca(2) sites with a charge balance occurring through sub-stitution of the fluoride ion by an oxygen ion. In parallel, in naturalapatite, it was shown that Eu’+ mainly occupies the nine-fold co-ordinated Ca(2) sites with charge compensation achieved with the in-troduction of an impurity Na+ ion in the neighbouring calcium sub-stitutional site [12]. In our Bi+-doped compounds, where Na+ hasnominally been introduced as a charge compensator, the above two Fig. 3. Emission decay curves at 300 K of Cas(PO4)3F-Bi+ (left, exc= 288 nm) and of Cas(VO4)3F-Bi (right, exc =325nm). situations remain open. At this stage, it is interesting to calculate theenvironmental factors he(Ca(1)) and he(Ca(2)) associated with the twosites to evaluate the nephelauxetic action occurring at these sites. Ac-cording to the original theory developed by Phillips and Van Vechten[13-15], such calculations are carried out on binary units to which theinitial host lattice must firstly be decomposed. The procedure for latticedecomposition in binary units is described in details in [3,16,17] andwill not be reproduced here for sake of brevity. The necessary crystal-lographic data for the decomposition (Wyckoff positions, interatomicdistances, coordination numbers, etc.) are obtained from the InorganicCrystal Structure Database and treated using VESTA software [18]. Then, for a given cation site (X) potentially occupied by Bi+ in ahost lattice, the environmental factor is obtained as: where fc(x-L) and a(x) and represent respectively the fractional cova-lency and the volume polarization of each chemical bond separatingcation X to its nearby ligands L (here oxygen) in the binary units. Thesummation runs to the coordination number Nx of cation X in the lat-tice.Q represents the effective charge carried by ligand L in each givenbinary unit. All details relative to the calculations can be found in [17]. The calculation gives he(Ca(1))=0.72 and he(Ca(2))=1.18. Fromthese values, we can estimate the energy EA(X, cm) of the A transitionof isolated Bi+ in either Ca(1) or Ca(2) sites using the empiricalequation introduced in [19]: This gives EA(Bi(1))==37,520cm(266nm)D and EA(Bi(2))= 29,850 cm(335nm), where Bi(1) and Bi(2) represent Bi’+ ionslocated in sites Ca(1) and Ca(2), respectively. It should be emphasizedhere that these values correspond to structural input data relative toregular Ca(1) and Ca(2) sites as present in undoped Cas(PO4)3F. Asstated above, incorporation of Bi+in Cas(PO4)3F should be compen-sated by Na defects if substitution occurs in Ca(1) sites and by anoxygen atom replacing the fluoride atom if substitution occurs in Ca(2)sites. This extra oxygen atom is not part of the(PO4) tetrahedra in thelattice and can move freely close to the trivalent dopant [11]. This willundoubtedly affect the values of he. It is difficult to recalculate thevalue of he for Bi(1)-Na(Ca) but we have tentatively recalculated it forBi(2)-O(F). For this, we have adapted the effective charge QL carried bythe ligand, maintain its coordination number at 3 and considered a Bi(2)-O(F) distance of 2.46 A (i. e. the sum of the ionic radii [11]). This 700 400 Fig. 4. Emission spectra of Cas(PO4)3F-Bi at 300 K recorded for delays of 4,25 and 200 usec after the laser pulse, exc= 288 nm. The delays after laser pulseare mentioned on the figure. gives he(Bi(2))-O(F))=1.43 with a corresponding EA(Bi(2)-O(F))=27,705 cm°(360nm). This value appears to be in good agreementwith the 354 nm excitation ascribed to Ca(2) sites in [6] and supportsthe attribution made in this paper for the violet emission band. Con-cerning our data in Fig. 1, none of the calculated energies of A transi-tion do match; the mismatch between experimental and calculated dataexceeds 2500 cm. This suggests that the observed luminescence fea-tures are in fact not due to isolated Bi3+. Instead of ascribing the 288 nm excitation to Bi+as done in [6], weestimate it more reasonable to ascribe this band to the presence of Bi+pairs. Basically, the presence of Bi+traces is not impossible in oxidiccompounds, including phosphates, although it has been shown by Penget al. [20] that Biis preferably stabilized in Sr+ or Ba+sites, not inCa+ sites. Further, Bi+ shows typical excitation features in the violet-blue region and beyond 500 nm for the lowest-lying one in correspon-dence with the crystal field splitted P3/2 level (P1/2-2P3/2 transi-tion). These features were not observed here. According to [21], elec-tronic transitions within Bi+pairs correspond to an intervalencecharge transfer (IVCT) of the type Bi(6s), Bi (6s)→ Bi++(6s),Bi+(6s"p). The energy of this transition has been formalized in [2] inthe following way: where XcN(Bi+) is the coordination-dependent electronegativity ofBi+taken from [22] and d’corr is the Bi+-Bi+ interatomic distancewithin the pair corrected for the doping effects. The correspondingvalue is defined as d'corr = dhost+1/2 [r(Bi+(X))+ r(Bi +(X’))-r(X)-r(X')], where X and X’ are the doping sites for the two nearby Bi+ionsin the host lattice. The first part of Eq. (3) relates to Bi+as the electrondonor while the second part relates to Bi+as the electron acceptor. Wewill consider in the following that the doping X relates to the donor andthe doping site X' relates to the acceptor. The coordination number atthe donor site is noted CN and that of the acceptor site CN’. In theinvestigated calcium fluoroapatites, X can be identical or different fromX’. dhost is the shortest distance separating these doping sites in thevirgin (undoped) host lattice and r(Bi), r(X) and r(X’) are the crystalradii of Bi+ and of the host cations X and X’ that are substituted toBi+. The other parameters are tabulated in [2] for coordinationnumbers CN varying from 5 to 12. It should be noted that only the casewhere X =X' (i.e. lattices in which the donor and acceptor occupy the Table1 Values for ad for the calculation of IVCT energies. CN↓ 5 6 7 8 9 10 12 CN'→ 5 2.57 2.62 2.68 2.74 2.76 2.81 2.87 6 2.47 2.52 2.57 2.63 2.65 2.70 2.75 7 2.35 2.41 2.46 2.51 2.53 2.58 2.63 8 2.26 2.30 2.36 2.41 2.43 2.47 2.52 9 2.22 2.27 2.32 2.37 2.39 2.43 2.48 10 2.14 2.19 2.24 2.29 2.30 2.35 2.39 12 2.06 2.10 2.15 2.20 2.21 2.25 2.30 Table 2 IVCT energies for Bi pairs in Cas(PO4)3F-Bi+depending on doping sites Ca(1,2). Values for Xcn(Bi) are taken from [1] and values for kcn are taken from[2]. Doping sites X'= Ca(1),CN'=9 X'=Ca(2), CN'=7 X=Ca(1),CN=9 d'corr =3.45 A d’corr =3.995 A X9(Bi +)=1.33 a;=2.39 a=2.32 k9 = 69,323cm IVCT =28,275 cm IVCT = 37,045 cm X=Ca(2),CN=7 d'corr =3.995 A d’corr =4.04 A x7(Bi+)=1.37 c=2.53 α²=2.46 kz=67,299cm IVCT=35,515cm IVCT =36,060cm same kind of site) was considered in this paper. In the present case, Xand X'may differ, which alters somehow the value of parameter acn.We therefore give in Table 1 the complete matrix of values ae for anykind of doping site X and X’. aare now used for acn in Eq. (3). InCas(PO4)3F-Bi+, four kinds of pairs can form depending on the dopingsites. The corresponding input data and IVCT energies calculated withEq. (3) are given in Table 2. The situation for Cas(PO4)3F-Bi+ is diagrammatically depicted inFig. 5. It comes that the energy of Bi(2)→Bi(1) CT reproduces rather wellthe position of the excitation band in Fig. 1, tolerating a mismatch of800 cmto the high energy side. Owing to the large Stokes shift as-sociated with the corresponding emission we conclude that there isgood chance that Bi(2)-Bi(1) pairs are involved in the luminescence ofCas(PO4)3F-Bi3+. We further note that the energy of Bi(2) →Bi(2) CTmatches well the excitation band reported in [5] (i. e. 36,630cm) forwhich the corresponding emission is Stokes shifted by a large amount aswell(≈18,000 cm°). This also suggests contribution from pairs insteadof isolated Bi+ in this case. At last, we note that the energy of Bi(1)→ Fig. 5. Energy level scheme of Cas(PO4)3F-Bi+ including experimental data(■) from the literature and from present work (*) and calculated data (O forintra-ionic A transitions of isolated Bi+; ▲ for intra-ionic A transitions ofisolated Bi(1-2)-Bi+(1-2)IVCT). Bi+ in Ca(1) and Ca(2) sites is labelledas Bi(1) and Bi(2), respectively. Bi(2)-O(F) indicates substitution of F to O forthe Ca(2) site, see text. Table 3 IVCT energies for Bipairs in Cas(VO4)3F-Bi+ depending on doping sites Ca(1-5). Values for Xcn(Bi) are taken from [1] and values for kcn are taken from [2]. Doping sites X'=Ca(1), CN'=7 X'=Ca(2), CN'= 7 X'=Ca(3), CN'=7 X'=Ca(4), CN'=8 X'=Ca(5),CN'=9 X=Ca(1), CN =7 d'corr =5.86 A d'corr =4.11A d’corr =4.08 A d'corr = 4.17 A d'corr =4.13 A x7(Bi+)=1.37 a²=2.46 α²=2.46 α=2.46 α;=2.51 x9=2.53 kz =67,299cm IVCT =53,495cm IVCT = 37,015 cm IVCT=36,610cm IVCT =37,920cm IVCT=37,370cm~ X=Ca(2), CN =7 d'corr =4.11 A d'corr =5.88 A d'corr =4.03 A dcorr =4.15 A d'corr =4.09 A xz(Bi+)=1.37 α}=2.46 α²=2.46 αz=2.46 α;=2.51 α=2.53 k7 =67,299cm IVCT=37,015cm IVCT = 53,625 cm IVCT=35,920cm~ IVCT=37,655cm IVCT=36,830cm X=Ca(3), CN =7 d’corr=4.08 A d'corr = 4.03 A d'corr=5.71 A d'corr =4.13 A d'corr =4.18 A X7(Bi+)=1.37 α7=2.46 α=2.46 α²=2.46 cx=2.51 9=2.53 k7 =67,299cm~ IVCT=36,610cm IVCT =35,920cm IVCT=54,480 cm IVCT=37,390cm IVCT=38,025cm X= Ca(4), CN =8 d’corr =4.15A d'corr =4.13 A d'corr =4.11 A d'corr =5.34 A d'corr =3.53 A X8(Bi+)=1.34 ag=2.36 ag=2.36 ag=2.36 αg=2.41 αg=2.43 kg=68,806cm IVCT=38,595cm IVCT=38,335 cm IVCT= 38,070 cm IVCT = 50,590cm IVCT=29,205cm X=Ca(5),CN =9 d’corr =4.13A d'corr=4.09 A d’corr =4.18A d'corr =3.53 A d’corr =5.67 A X9(Bi十+)=1.33 αg=2.32 αg=2.32 α=2.32 c8=2.37 a=2.39 kg =69,323cm~ IVCT = 38,850cm IVCT = 38,330cm IVCT = 39,490 cm IVCT=29,830 cm IVCT = 53,335 cm Bi(1) CT coincides with the 354 nm excitation found in [6], but the verysmall Stokes shift associated with the corresponding emission makes theattribution of this feature to pairs less probable. 4.2. Case study of Cas(VO4)3F-Bi+ The monoclinic crystal structure of Cas(VO4)3F contains five cationsites Ca(1-5) available for Bi+, three V(1-3) sites, 12 O(1-12) sitesand 1F(1) site. Sites Ca(1-3) are coordinated with 6 oxygen and onefluoride atoms and resemble site Ca(2) in Cas(PO4)3F. Sites Ca(4-5)are respectively eight and nine-coordinated with oxygen atoms andresemble more sites Ca(1) in Cas(PO4)3F. All V(1-3) sites are tetra-hedrally coordinated. The calculation of the environmental factors forthe Ca(1-5) sites gives he(Ca(1))=1.17, he(Ca(2))=1.20,he(Ca(3)=1.00, he(Ca(4)=0.87 and he(Ca(5) =0.87 with correspondingenergies of the A transition: E (Bi(1))=29,960 cm°(337nm), E (Bi(2))=29,640 cm°(337 nm),EA(Bi(3))=32,120cm(311nm), EA(Bi(4)) =34,290 cm(291nm) and E (Bi(4))=34,290cm(291nm).Substituting the fluoride atom to an oxygen atom for sites Ca(1-3) inthe same way as done in Cas(PO4)3F-Bi+rises: he(Bi(1))-O(F))=1.25,he(Bi(2))-O(F))=1.28 and he(Bi(3))-O(F))=1.09 with correspondingEA(Bi(1)-O(F))=29,150cm(343nm), EA(Bi(2)-O(F))=28,875 cm°(346 nm)and EA(Bi(3)-O(F)) =30,890 cm°(323nm). Dealing nowwith charge transfer processes (MMCT or IVCT) that may occur in Cas(VO4)3F-Bi+, we compile in Table 3 the necessary input data to runEq. (3) and in Table 4 those to run the following Eq. (4) that relates tothe Bi+→一5+ MMCT energy [1]: All parameters in this equation have the same meaning as in Eq. (3)but the value of kcy andac differ. They are tabulated in [1]. dcorr is asimplified version of d'corr in which only one doping site X with co-ordination number CN is considered for Bi+(the donor site). The ac-ceptor site is that of V5+ with coordination number CN'=4. Then wehave dcorr =dhost+1/2 [r(Bi)-r(X)], with X= Ca(1-5) in the pre-sent case. The situation for Cas(VO4)3F-Bi+ is depicted in Fig.6. We find that regular A transition in Bi(1)-O and Bi(2)-O, on onehand and Bi(4)→ Bi(5) IVCT,Bi(1))→V(1) and Bi(3))→V(3) MMCT onthe other hand possess an excitation energy close to 29,000 cm",i. e.≈1600 cm higher than the 365 nm (27,400 cm) excitation bandfound in Fig. 2(c). It is difficult, at this stage, to individuate with betteraccuracy the exact nature of this excitation state. We note further thatisolated Bi(4-5)-O and several IVCTs and MMCTs can contribute to theexcitation features located in the spectral range 35,000-38,000 cm inFig. 2 and that isolated Bi(3)-0 together with Bi(4)→V(1) or Bi(5)-V MMCT energies in Cas(VO4)3F-Bi’+ depending on doping sites Ca(1-5) for Bi’+ donors and V(1-3) sites for v5+ acceptors. Values for XcN(Bi’+),x4(V+)k and a taken from [1]. Acceptor sites → V(1),CN'=4 V(2), CN'=4 V(3), CN'=4 Donor sites ↓ X4(V+)=2.46 x4(V+)=2.46 x4(V+)=2.46 X=Ca(1), CN =7 d’corr =3.13A d’corr=3.69 A d’corr =3.46A x7(Bi+)=1.37 α²=1.02 a=1.02 α²=1.02 kZ=51,095 cm MMCT=29,040cm MMCT= 35,255 cm MMCT= 32,945 cm X=Ca(2), CN =7 d’corr =3.40A d’corr =3.13 A d’corr =3.64 A x7(Bi+)=1.37 αl=1.02 α=1.02 aZ=1.02 kZ=51,095 cm MMCT = 32,290cm MMCT = 29,040 cm MMCT = 34,780cm X=Ca(3),CN =7 d'cor=3.71A d'corr =3.32 A d'corr=3.16 A X7(Bi +)=1.37 ²=1.02 αZ=1.02 =1.02 kZ=51,095 cm MMCT =35,440cm MMCT =31,385cm MMCT =29,430cm X=Ca(4), CN =8 dcorr =3.255A dcorr =3.485 A d'corr =3.275 A X8(Bi +)=1.34 α=0.99 α;=0.99 =0.99 k=52,239cm MMCT=30,915cm MMCT = 33,495 cm MMCT = 31,155 cm- X=Ca(5), CN=9 d'corr = 3.385 A d'corr =3.275 A d'corr =3.395 A X9(Bi +)=1.33 α2=0.99 α2=0.99 α2=0.99 k2=52,631cm MMCT=32,130cm- MMCT =30,860cm MMCT=32,245 cm Fig. 6. Energy level scheme of Cas(VO4)3F-Bi+ including experimental data(■) and calculated data (● for intra-ionic A transitions of isolated Bi+(1-5)-V5+(1-3) MMCT and▲ for Bi+(1-5)-Bi+(1-5) IVCT). The dotted linemarked (VO4)indicates the spectral region corresponding to host vanadateexcitation. (2)MMCTs fall in the spectral region of (VO4)excitation. Again, thisconcomitance of degenerated excitation states with different originsmakes it difficult to describe the luminescence process with certainty inCas(VO4)3F-Bi+. This issue can be, at least for a part, solved by ap-plying external stresses like pressure to lift this degeneracy [4] and /orby developing methods allowing to predict the Stokes shifts associatedwith the different categories of emitters [3]. This is under progress. 5. Conclusion The purpose of the present work was to demonstrate how complexthe Bi+spectroscopy can be in inorganic solids and that the attributionof the luminescence features to isolated Biions as an obvious optionis not always relevant. Thereby, in the calcium fluorophosphates pre-pared in this work, the luminescence can be very satisfactorily inter-preted in terms of charge transfer processes, either within Bi +-Bi+pairs in Cas(PO4)3F-Bi++ or within Bi +-Bi+and Bi+-v+pairs inCas(VO4)3F-Bi+. Acknowledgements This research did not receive any specific grant from fundingagencies in the public, commercial, or not-for-profit sectors. ( [1] P . Bo u tinaud , R e v i s itin g t he s p e c tr o s c opy o f t h e Bi i on i n o x ide c om p o u nds , I n o r g . Che m . 5 2 ( 201 3 ) 6 028- 6 0 3 8. ) ( [2] 1 P. Boutinaud , O n t h e l u min e s c enc e of B i + p a i rs i n oxidi c co mpoun d s, J . L u m i n. 1 97 (20 1 8) 228-232. ) ( [3] M . A me r , P. B o u tinau d , On the c har a cte r o f t h e o p ti c al tr a n s itions in c l o s ed-shel l t r a n s it i o n m et a l o x i d e s do pe d with B i + , Ph ys . C h em. C h em . P h y s . 19 ( 2 0 1 7) 2591- 2 596 . ) ( [4] S . M ahlik, M . A mer, P . B outinaud, E n e rgy Le v el S t r u c ture o f Bi+i n Z i rco n and S c h ee l i t e P ol y morphs of YVO4 , J . P hys . Che m. C 1 20 ( 2016) 8261 - 82 6 5. ) ( [ 5] H. Z hu , Z. X i a , H . L i u , R . M i, Z. H ui, L uminescence p ro p er t ie s and e n erg y tra ns fe r of B i +/ E u +- c o d oped C a 10( P O4)6 F 2 ph o s p hor s, Ma t e r. R es . Bu l l. 4 8 ( 2 0 13) 3 513- 3 51 7 . ) ( [ 6] C . L i, Z. S o n g, J. Q i u , Y . L i , Q. W a n g , Z. Ya ng , Z. Y i n , Y . Y ang , Broa d b a nd o ran ge e m is s i o n f rom B i activ a te d c a lc i u m fl u o r o p hospha t es, Mater. Re s. B ul l. 50 ( 2014 ) 4 90- 4 93. ) ( [7]E . C a v a l li ,F . An g i u l i , F . M ez z a d r i , M . T r e v i sani , M.B e t ti ne ll i , P . B o u ti n a u d , M . B r ik, T unab le l uminesce n ce o f B i3+ -d o p ed Y P xV 1 -x 04 ( 0 ≤ x ≤ 1 ) , J . P h y s: Co n d e n s. M atter 2 6 ( 2 01 4 ) 3 85 5 03. ) ( [8] L . H . B rixn e r, C r y st al g ro w t h a n d fl u or e sc ent p r op e rt i e s of r ar e - ea r t h dope d ca l c i u m fluoro v anadate, J . S o lid Stat e Chem . 1(1970) 1 85-189. ) ( [9] ( G . B la s se , In f lu e nce of lo c a l c h a r g e compe n sation o n s it e o ccu pat i o n a nd lu m i n e s- c en c e o f a pa t it es, J. S o l i d S t a t e Che m. 14 (19 7 5 ) 1 8 1- 1 84 . ) ( [10] B . P i rio u, D . F a h m i , J . D exper t - Ghy s , A. Ta i t a i, L.J. L a cout, U nu s ual fl u o resce n c e p r o per t i e s of E u ’ in ox y apa ti tes, J . L umin . 3 9 (1 9 87) 97 - 10 3 . ) ( [11] R . S a h oo, S . K . B h at tach a r ya , R . D ebnath,A n ew ty p e of cha r ge co m p en s atin g me - ch a n is m i n C a 5(P O 4) 3 F :Eu+ ph o s pho r , J. Soli d St a t e C h e m 1 7 5 (20 0 3 ), p p . 21 8 - 2 25. ) ( [ 12] M . G af t , R . Re i s fe ld , C . Panczer, S . Sh ova l , B . Cha mp agn on , G . B o u lo n , E u +l u - m ines c e nc e i n h i gh -sy m m etr y s it e s of n a t u r a l a p a tite, J . L um in 5 7 2- 5 74 (1997) 7 2- 7 4. ) [13] J.C. Phillips, Dielectric definition of electronegativity, Phys. Rev. Lett. 20 (1968)550-553. ( [14] J J . A . V a n V ec hten, Q u an t u m die l ec t r i c t h e o r y of e l ec t ro nega t i v it y i n c o val e nt ) ( s ystems.I . E l e c t ro nic d i ele ct ric c o n s tan t , P h ys . R e v . 18 2 (19 69) 89 1 -905 . ) [15] J.A. Van Vechten, Quantum dielectric theory of electronegativity in covalentsystems.II. Ionization potentials and interband transition energies, Phys. Rev. 187(1969) 1007-1020. ( [ 1 6] J. S. S hi , Z.J . W u, S . H . Z h ou, S . Y . Zh a ng , De p e ndence of cr yst al f i e l d sp l i t t i n g o f 5d l e ve ls o n h osts i n t he h a l ide c r y stal s , C h e m. P h ys . Le tt. 3 8 0 ( 20 0 3) 2 45 . ) [17]J.S. Shi, S.Y. Zhang, Barycenter of energy of lanthanide 4f-15d configuration inofIinorganic crystals, J. Phys. Chem. B 108 (2004) 18845-18849. ( [ 18] K . M omma, F. I z u m i , V E S TA : a t h r e e -d im e n s iona l vi s u a l iz ati o n s y s tem for e l e c - tronic a n d s tructur a l a n al y si s , J . Appl. C r y s t allogr. 4 1 ( 2 008) 6 5 3 - 65 8 . ) [19]L. Wang, Q. Sun, Q. Liu, J. Shi, Investigation and application of quantitative re-lationship between sp energy levels of Biion and host lattice, J. Sol. State Chem.191 (2012) 142-146. [20] 1M. Peng, J. Lei, L. Li, L. Wondraczek, Q. Zhang, J. Qiu, Site-specific reduction ofBi3+ to Bi2+ in bismuth-doped over-stoichiometric barium phosphates, J. Mater.Chem. C. 1 (5303) (2013). ( [21] R . H . P . A water , P . D oren b o s, T he B i 6 s a nd 6 p elec t ron b i nding ener g i es i n r e - l at i o n to t h e c hemi c al e n vironment o f i nor ga nic c o mpou n ds, J . L u min. 18 4 (20 1 7 ) 2 21- 2 31. ) [22] K. Li, D. Xue, Estimation of electronegativity values of elements in different valencestates, J. Phys. Chem. A 110 (2006)11332-11337. The spectral and dynamical properties of Bi3+ in the calcium fluorophosphates Ca5(PO4)3F and Ca5(VO4)3F are investigated at room and liquid nitrogen temperature. The data are interpreted at the light of recent semiempirical models that allow calculating the energy of A-like (isolated Bi3+), D-like (metal-metal charge transfer) and pair-like transitions from the knowledge of the crystal structure of the host lattices. It is concluded that the attribution of the luminescence features to isolated Bi3+ ions is not always an obvious option and that theluminescence can also be very satisfactorily interpreted in terms of charge transfer processes, either within Bi3+-Bi3+ pairs in Ca5(PO4)3F-Bi3+ or within Bi3+- Bi3+ and Bi3+-V5+ pairs in the newly reported Ca5(VO4)3F -Bi3+.
关闭-
1/6

-
2/6
还剩4页未读,是否继续阅读?
继续免费阅读全文产品配置单
北京欧兰科技发展有限公司为您提供《掺杂Bi3的氟化钙磷灰石中激光诱导光谱检测方案(激光产品)》,该方案主要用于非金属矿产中激光诱导光谱检测,参考标准《暂无》,《掺杂Bi3的氟化钙磷灰石中激光诱导光谱检测方案(激光产品)》用到的仪器有Ekspla NT340 高能量可调谐激光器(OPO)。
我要纠错
相关方案






 咨询
咨询




