方案详情文
智能文字提取功能测试中
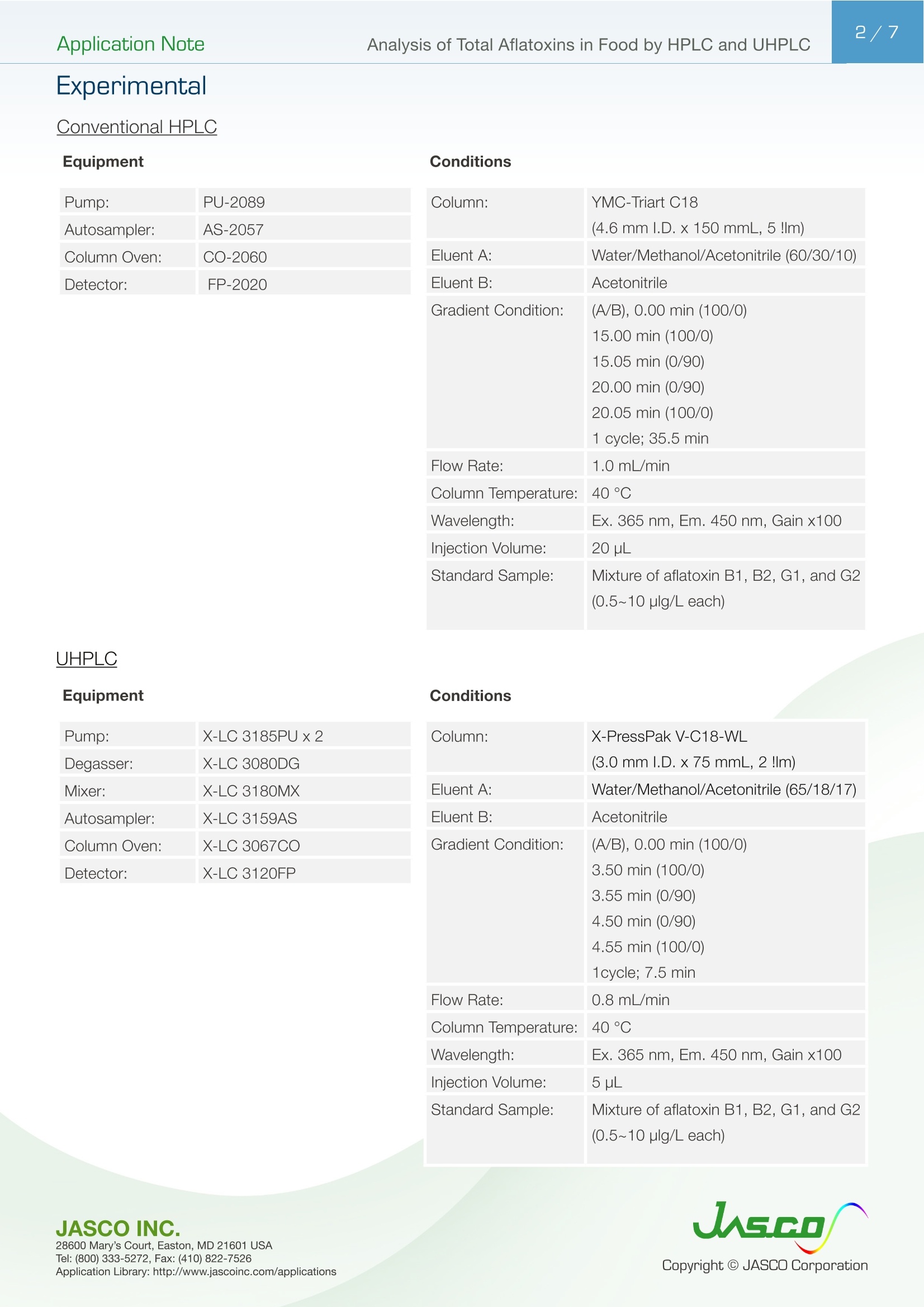
黄曲霉毒素是由生活在热带或亚热带地区的黄曲霉、寄生曲霉菌和诺米曲霉菌等微生物产生的一组真菌毒素,具有很强的致癌作用。据报道,在包括水果、谷物和香料在内的各种食品中,经常发现黄曲霉毒素含量较高,超过了美国食品药品监督管理局等食品安全机构规定的安全水平。现行法规要求黄曲霉毒素总量(黄曲霉毒素B1、B2、G1和G2的总和)必须低于10μg/kg。柱前衍生方法使用三氟乙酸(TFA)与高效液相色谱法结合荧光检测,大大提高了黄曲霉毒素B1和G1的灵敏度。除了衍生方法外,还可以使用多功能柱或免疫亲和柱来提高样品制备过程中的再现性和回收率。Application Note 2/7 Analysis of Total Aflatoxins in Food by H PLC and UHPL C Af l atoxins are a group of mycotoxins produced by microorg a nisms such as Aspergillus flavus, Aspergi l lus parasit i cus a n d Aspergi l lus nom i us l i v i ng in tropical or subt r opical r egions an d have a strong carcinogenic affect . It has been reported that higher levels of aflatoxins are often found which exceed the safe levels lai d down by food safety agencies such as the FDA i n wide variety of food products i ncluding f ruits,grains and spices. Present regulations require t h a t total aflatoxins (sum of aflatoxin B1, B2, G1 and G2) mu s t be lower than 10ug/kg. A preco l umn derivatiza t ion method that uses trifluoroacetic acid (TFA) in combination with HPLC with fluorescence detection offers greatly improved sensitivity for aflatoxins B1 and G1. In addition to the d e rivatization method, the ut il ization of mul t i-functional columns or immunoaf f inity columns can be used to improve the reproducibility and recovery i n the sample preparation procedure. JASCO I NC. Applic a tio n Libra r y: htt p ://www.jascoinc .com/applicat i ons A n al ysi s o f T o t a l A f la t o x i n s in F o od by H P L C an d UH P L C Application Note E xp e rimenta l C onventiona l HPL C Pump: PU-2089 Autosampler: AS-2057 Column Oven: CO-2060 Detector: FP-2020 Column: YMC-Triart C18 (4.6mm l.D.x 150 mmL, 5 !lm) Eluent A: Water/Methanol/Acetonitrile (60/30/10) Eluent B: Acetonitrile Gradient Condition: (A/B), 0.00 min (100/0) 15.00 min (100/0) 15.05 min (0/90) Flow Rate: 1.0 mL/min Column Temperature: 40°C Wavelength: Ex. 365 nm, Em. 450 nm, Gain x100 Injection Volume: 20 uL Standard Sample: Mixture of aflatoxin B1, B2, G1, and G2 (0.5~10 plg/L each) UHPLC Equipment Conditions P u m p :X-LC 3185PUx2Column:X-PressPak V-C18-WL Degasse r :X-LC 3080DG (3.0 mm I.D.x75 mmL, 2 !lm)Mixer:X -LC 3180MX Eluent A:Water/Methanol/Acetoni t rile (65/18/17)Autosampler:X -LC 3159ASEluent B:Acetonitr i le Column Oven :X-LC 3067CO Gradient Condit i on:(A/B), 0.00 mi n (100/0)Detector:X-LC 3120FP 3.50 min (100/0)3.55 min (0/90)4.50 min (0/90)4.55 m i n (100/0)1cyc l e; 7.5 min F l ow Rate:0.8 mL/min Col u mn Temperature :40°C Waveleng t h:Ex. 365 nm, Em. 450 nm, Gain x100Inj e ctio n Volume:5 pL Standard Sample :Mixt u re of afla t oxin B 1, B 2, G1, and G2(0.5~10 p l g/L each) Application Note Structure Aflatoxin B1, B2, G1 and G2 are compound s that yield nat i ve fluorescence. However, the fluorescence i ntensity of B1 and G1 is much less i n comparison to that of B2 and G2 and accordingly, the sensitiv i ty of B1 and G1 must be improved by changing f rom the natural form i nto a hydroxidized form using TFA der i vatization. The structure of aflatoxin B1, B2, G1 and G2 and the structure of derivatized B1 a n d G 1 are shown in f igure. 1. Fi g u r e 1. St ruc t u re o f a f la toxin B 1, B2, G1, G2 a n d TFA-d e r iv a t iz ed B1 an d G 1 De ri v a t i z ation The procedures for TFA derivat i zation of the standard m i xture of aflatoxins and actual sample are shown i n Figure. 2. Fig u re . 2. TF A d e ri va t iza t io n 1.0 mL of standard mixture of aflatoxins (each of 0.5, 1.0, 5.0 and 10 pg/L) Or actual sample solution, purified 0.1 mL TFA Sample enrichment by so l vent e l imin a tion usi n g d ry nitrog e n gas at less than 45℃. 0.9 mL Milli -Q water/acetonitri l e (90/10) Plug the vessel and mix thoroughly. Stand in a dark place at room temperature fo r 15 m in. *1 A f ter mixing, inject an aliquot of the sample into the HPLC (Conventional HPLC: 20 pL, UHPLC: 5pL) *1 I n orde r t o a v oi d t he ads o r pt i on of a f latoxins onto t he inner wal l of vesse l , t h e vessel must be ri nsed w i t h aceton i tri l e and Milli -Q wate r, t h en d r ied. A p plicat i o n L i br a r y : htt p ://www.j as co i n c .c o m /ap p l i c a ti o n s Application Note S amp le Pr epa ra tio n Corn grits and roasted peanuts were selected as test samples, and a multi -f unctional column was used for the sample preparation of corn grits, while an immune -aff i nity column was used for the preparation of roasted peanuts . The sample preparation procedures are shown in Fig. 3. 1. Co r n Grits Extraction 10.0 g of corn gr i ts 40 mL a c etonitrile/Milli-Q water (90/10)*1 Mix/shake for 30 minutes Centrifuge (4000 rpm, 10 min) The extract will be in t he supernatant . Purification 6 mL of supernatant Apply the supernatant to a multi -func t iona l column (MycoSep 226 AflaZon +*2) Fractionate the f i rst 2 mL of eluent as a purified so l ution with the f low rate at 1 mL/min. Apply p urified solution to the TFA derivatizaton 2. Roasted Peanuts Extraction 20.0 g of roasted peanuts ground and homogenized 40 m L acetonitrile/Milli -Q water (90/10)*1 Mix/shake for 30 minutes Put 10 mL of supernatant into a 50 mL measuring f lask and make up the volume with Mi l l i-Q water to 50 mL F i lter the solution using glass wool The ext r act will be in t h e eluent *1 I n t he rec o very es t im a t i on t est , t h e st and a rd m ixt ure of a fl a toxins (each o f 0.5 11g/L) in a c ceton i t r il e/Milli-Q water (90/10) will be u se d . *2 Th r ee s epar a t i o n m od es (r e ver se ph as e , n o rma l ph a s e a nd i o n -e x c hange) are av a il a bl e i n the m ult i -f u nc t i on al c o lum n .(R o m er L abs) *3 Imm un oaf f inity c o l u mn (HORIBA) u t i lizing u niqu e bin d ing cap a bil ity be tw e e n a nt i-a f l a t o x in m ono clo nal an ti-b o dy a nd a flatoxins *4 Le t t he co l um n e q uilib r a t e to room t em p e r a t u r e. Punch a h o l e on t h e upp e r c ap c a re fully so t h a t th a t t h e a i r wou l d not p a ss into t h e g e l a nd t h en ta ke t h e u ppe r a nd t h e l o w er c ap . *5 Mak e up t h e Milli -Q wat e r s olut i o n wit h 0.20 g potassi um c hlorid e,0.20 g o f po ta s s iu m d ih yd r oge n phos ph a te, 1.16 g of dis od i u m h y drog e n ph o spha te a n d 8.0 g o f sodium ch l oride (pH 7.4) to 1L . *66Flush t h e colu mn ca r efu l ly so t h a t t h e a ir wil l not pass i n to the ge l. *7 Min imi ze the wa t e r content o f t h e final e l uent in t h e ace to ni t ri l e. If t h e wa t er conte n t i s r ela t iv el y h i gh in the fi n a l elut i on , i t wi ll ta k e a l onge r fo r solvent el im ina t ion, wh i ch may c a use dena t uratio n of afla t ox i ns . *8 T o r elease t h e i n teracted af l atoxin s from g el comple t ely. Purification Drain the preservation solvent from immune-affinity column (AFLAKING*3)*4. F l ush the column with 3 mL of PBS.*5,*6 Apply 10 mL of el u en t onto the column at flow rate of 1 drop/sec.*6 Flush the column with 3 mL PBS, twice.*6 F l ush the column with 3 mL Milli -Q water, twice .*6 Inject air into the column to completely drain the water out of column.*7 Apply 1mL of acetonitr i le onto the column by dripping and collect the eluent . Leave the column for 5 minutes.*8 Apply 2 m L of acetonit r ile onto t h e column by dripp i ng and, collect the elu e nt Inject air into column to drain the acetonit ri le out of column and collect a total of 3 m L acetonit r ile solution as the purified solution. Apply the purified solution to the TFA deriv a tizaton (Fig.2.) R e s u l ts The chromatograms of standard mixtures of TFA-derivat i zed aflatoxins (each of 5.0pg/L ) are shown in the figure 4 [by conventional HPLC (upper), by UHPLC (l ower )]. The separat i on is completed within 12 minutes by conventional HPLC and within the 3.5 minutes by the UHPLC. The linearity of t he standard mixtures of aflatoxins is in t he range 0.5 to 10pg/L with excellent correlation achievedr>=0.9997 for both conventiona l HPLC and UHPLC. Good reproducibi l ity including the TFA derivatization procedure (N =6) was obtained for the conventional HPLC of better than 0.2% RSD and 3% RSD in peak retention time and area , respectively . The UHPLC also yie l d e d good reproduc i bility with better than 0.2% RSD and 3.5% RSD in peak retention t i m e and area,r espectively. The 1.0ug/L of standard m i xture of a flatoxins was used for this est i mation. Conventional HPLC UHPLC Fig ur e. 4. C h ro m atog ra ms o f s t and a rd m ix t u re o f a f la t o xi ns (5.0 pg/L ea c h, TFA d e ri v a tiz ed )1=A fl a t o xin G1, 2=A f la toxin B1, 3=Afl a t o xin G2, 4=Af l a toxin B2 Chromatograms of corn grits samples prepared using the multi-functional columns (MycoSep 226AflaZon+)in the sample preparat i on are shown in f igure 5 (both the conventional HPLC and UHPLC). The samples from corn grits and those spiked with a sta n dard mixture o f aflatoxins were used for the recovery es t imation of af l atoxins . Almost no contaminant peaks are observed i n the chromatograms and good recovery of standard aflatoxins was obtained for both con v entional HPLC and UHPLC as shown in Table 1. Chromatograms of the ro a sted peanuts sample prepared using the i mmunoaf f inity column (AFLAKING) in sample preparation are shown i n figure 6 (both the conventional HPLC and UHPLC). The samples from roasted peanuts and those sp i ked with the st a ndard mixture o f af l atoxins were u s ed for th e recovery estimation of aflatoxins . Almos t n o contaminant peaks are observed i n the chromatograms and good recovery of standard aflatoxin was obtained for both conventional HPLC and UHPLC as shown in Table 2. Fig ur e 5. Chromatog r ams o f pu r if i ed solution fr o m corn gri ts 1=Aflatoxin G1, 2=Af l atoxin B 1, 3=A f latoxin G 2, 4=Af l ato x in B 2 Figure 6. Chrom a tograms o f p ur i f i ed so l ut i on f r o m r o as t ed p ea nut s 1=A f l atoxi n G 1, 2=A f l a t o x i n B1, 3=A f l atoxi n G2, 4=A fla t oxi n B2 Table 1. Recovery [%] of s t andard af l atoxins Conventional UHPLC Conventional UHPLC HPLC HPLC 107 108 101 99 Aflatoxin B1 106 102 99 98 Aflatoxin G2 100 100 102 104 Aflatoxin B2 100 100 101 102 A p plicat i o n L i br a r y : htt p ://www.j as co i n c .c o m /ap p l i c a ti o n s
关闭-
1/7

-
2/7
还剩5页未读,是否继续阅读?
继续免费阅读全文产品配置单
佳士科商贸有限公司为您提供《食品中黄曲霉毒素的高效液相色谱和超高效液相色谱仪分析 JASCO》,该方案主要用于生物药品原料中黄曲霉毒素检测,参考标准《暂无》,《食品中黄曲霉毒素的高效液相色谱和超高效液相色谱仪分析 JASCO》用到的仪器有JASCO高效色谱仪LC-4000、JASCOFP-8000系列荧光光谱仪。
我要纠错
推荐专场
相关方案









 咨询
咨询