方案详情文
智能文字提取功能测试中
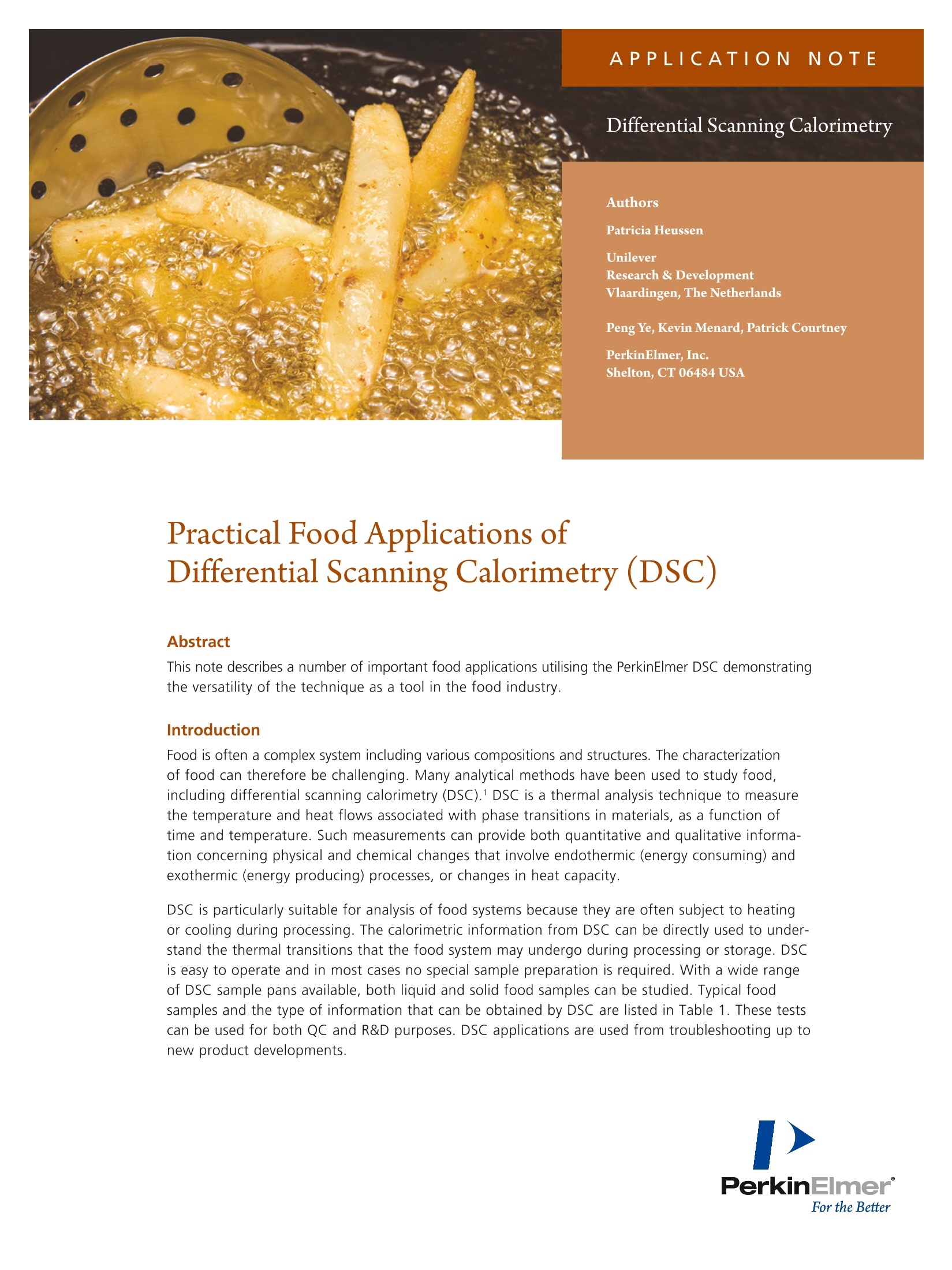
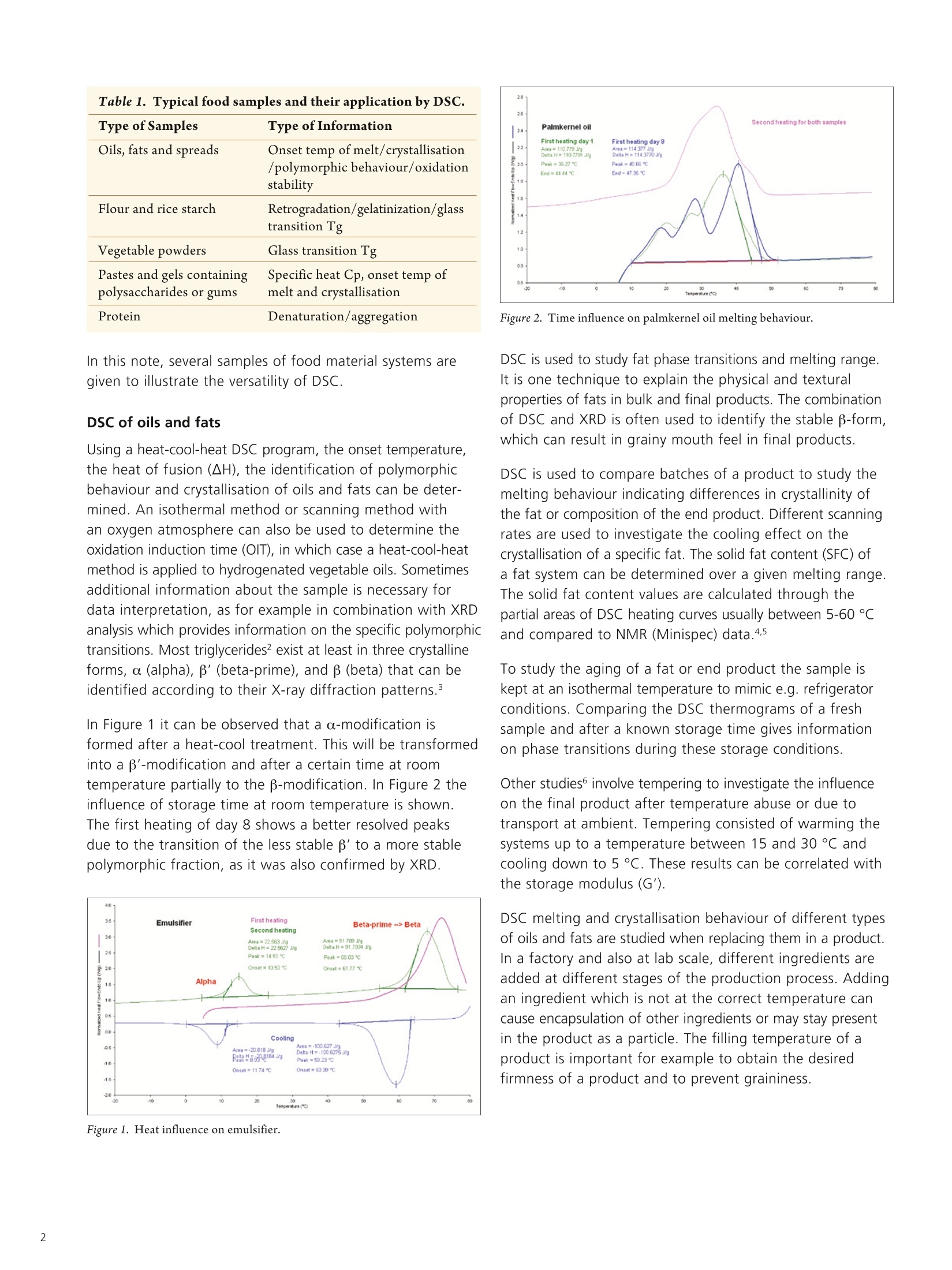
009742_01 APPLICATIONNOTE Differential Scanning Calorimetry Authors Patricia HeussenUnileverResearch & DevelopmentVlaardingen, The Netherlands Peng Ye, Kevin Menard, Patrick Courtney PerkinElmer, Inc.Shelton, CT 06484 USA Practical Food Applications ofDifferential Scanning Calorimetry (DSC) Abstract This note describes a number of important food applications utilising the PerkinElmer DSC demonstratingthe versatility of the technique as a tool in the food industry. Introduction Food is often a complex system including various compositions and structures. The characterizationof food can therefore be challenging. Many analytical methods have been used to study food,including differential scanning calorimetry (DSC).1 DSC is a thermal analysis technique to measurethe temperature and heat flows associated with phase transitions in materials, as a function oftime and temperature. Such measurements can provide both quantitative and qualitative informa-tion concerning physical and chemical changes that involve endothermic (energy consuming) andexothermic (energy producing) processes, or changes in heat capacity. DSC is particularly suitable for analysis of food systems because they are often subject to heatingor cooling during processing. The calorimetric information from DSC can be directly used to under-stand the thermal transitions that the food system may undergo during processing or storage. DSCis easy to operate and in most cases no special sample preparation is required. With a wide rangeof DSC sample pans available, both liquid and solid food samples can be studied. Typical foodsamples and the type of information that can be obtained by DSC are listed in Table 1. These testscan be used for both QC and R&D purposes. DSC applications are used from troubleshooting up tonew product developments. Table 1. Typical food samples and their application by DSC. Type of Samples Type of Information Oils, fats and spreads Onset temp of melt/crystallisation /polymorphic behaviour/oxidation stability Flour and rice starch Retrogradation/gelatinization/glass transition Tg Vegetable powders Glass transition Tg Pastes and gels containing Specific heat Cp, onset temp of polysaccharides or gums melt and crystallisation Protein Denaturation/aggregation In this note, several samples of food material systems aregiven to illustrate the versatility of DSC. DSC of oils and fats Using a heat-cool-heat DSC program, the onset temperature,the heat of fusion (AH), the identification of polymorphicbehaviour and crystallisation of oils and fats can be deter-mined. An isothermal method or scanning method withan oxygen atmosphere can also be used to determine theoxidation induction time (OIT), in which case a heat-cool-heatmethod is applied to hydrogenated vegetable oils. Sometimesadditional information about the sample is necessary fordata interpretation, as for example in combination with XRDanalysis which provides information on the specific polymorphictransitions. Most triglycerides? exist at least in three crystallineforms, α (alpha), '(beta-prime), and β (beta) that can beidentified according to their X-ray diffraction patterns.3 In Figure 1 it can be observed that a o-modification isformed after a heat-cool treatment. This will be transformedinto a B'-modification and after a certain time at roomtemperature partially to the B-modification. In Figure 2 theinfluence of storage time at room temperature is shown.The first heating of day 8 shows a better resolved peaksdue to the transition of the less stable p'to a more stablepolymorphic fraction, as it was also confirmed by XRD. Figure 2. Time influence on palmkernel oil melting behaviour. DSC is used to study fat phase transitions and melting range.It is one technique to explain the physical and texturalproperties of fats in bulk and final products. The combinationof DSC and XRD is often used to identify the stable B-form,which can result in grainy mouth feel in final products. DSC is used to compare batches of a product to study themelting behaviour indicating differences in crystallinity ofthe fat or composition of the end product. Different scanningrates are used to investigate the cooling effect on thecrystallisation of a specific fat. The solid fat content (SFC) ofa fat system can be determined over a given melting range.The solid fat content values are calculated through thepartial areas of DSC heating curves usually between 5-60 ℃and compared to NMR (Minispec) data.4.5 To study the aging of a fat or end product the sample iskept at an isothermal temperature to mimic e.g. refrigeratorconditions. Comparing the DSC thermograms of a freshsample and after a known storage time gives informationon phase transitions during these storage conditions. Other studies involve tempering to investigate the influenceon the final product after temperature abuse or due totransport at ambient. Tempering consisted of warming thesystems up to a temperature between 15 and 30 °C andcooling down to 5 C. These results can be correlated withthe storage modulus (G'). DSC melting and crystallisation behaviour of different typesof oils and fats are studied when replacing them in a product.In a factory and also at lab scale, different ingredients areadded at different stages of the production process. Addingan ingredient which is not at the correct temperature cancause encapsulation of other ingredients or may stay presentin the product as a particle. The filling temperature of aproduct is important for example to obtain the desiredfirmness of a product and to prevent graininess. An AOCS’ method can be carried out for quality control of fatsto analyse these raw materials used in food products. This isa "fingerprint" method whereby the sample is melted, subse-quently cooled down with a predefined scanning rate to a lowtemperature. After crystallisation for a specific time, a heatingcurve is obtained also with a predefined scanning rate. DSC of starch samples Starch8.9, a major structure-forming food hydrocolloid10,is a polymeric mixture of essentially linear (amylose) andbranched (amylopectin) molecules. Small amounts of non-carbohydrate constituents (lipids, phosphorus, and proteins)present in native starch also contribute to its functionality.Starch is used as thickening agent in e.g. dry sauce bases,instant soups, mayonnaise, spreads. Starch pastes can beused as stabilizers for oil emulsions in for instance dressings. Native starch or modified starch used in these types of foodproducts can show different endothermic peaks in the DSCthermograms respectively, retrogradation (recrystallizedamylopectin), gelatinization (50
-
1/4
-
2/4
还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
珀金埃尔默企业管理(上海)有限公司为您提供《植物油中量热检测方案 》,该方案主要用于食用植物油中理化分析检测,参考标准《暂无》,《植物油中量热检测方案 》用到的仪器有差示扫描量热仪PerkinElmer DSC8500 。
我要纠错
推荐专场
差示扫描量热仪(DSC/DTA)
更多相关方案






 咨询
咨询


