方案详情文
智能文字提取功能测试中
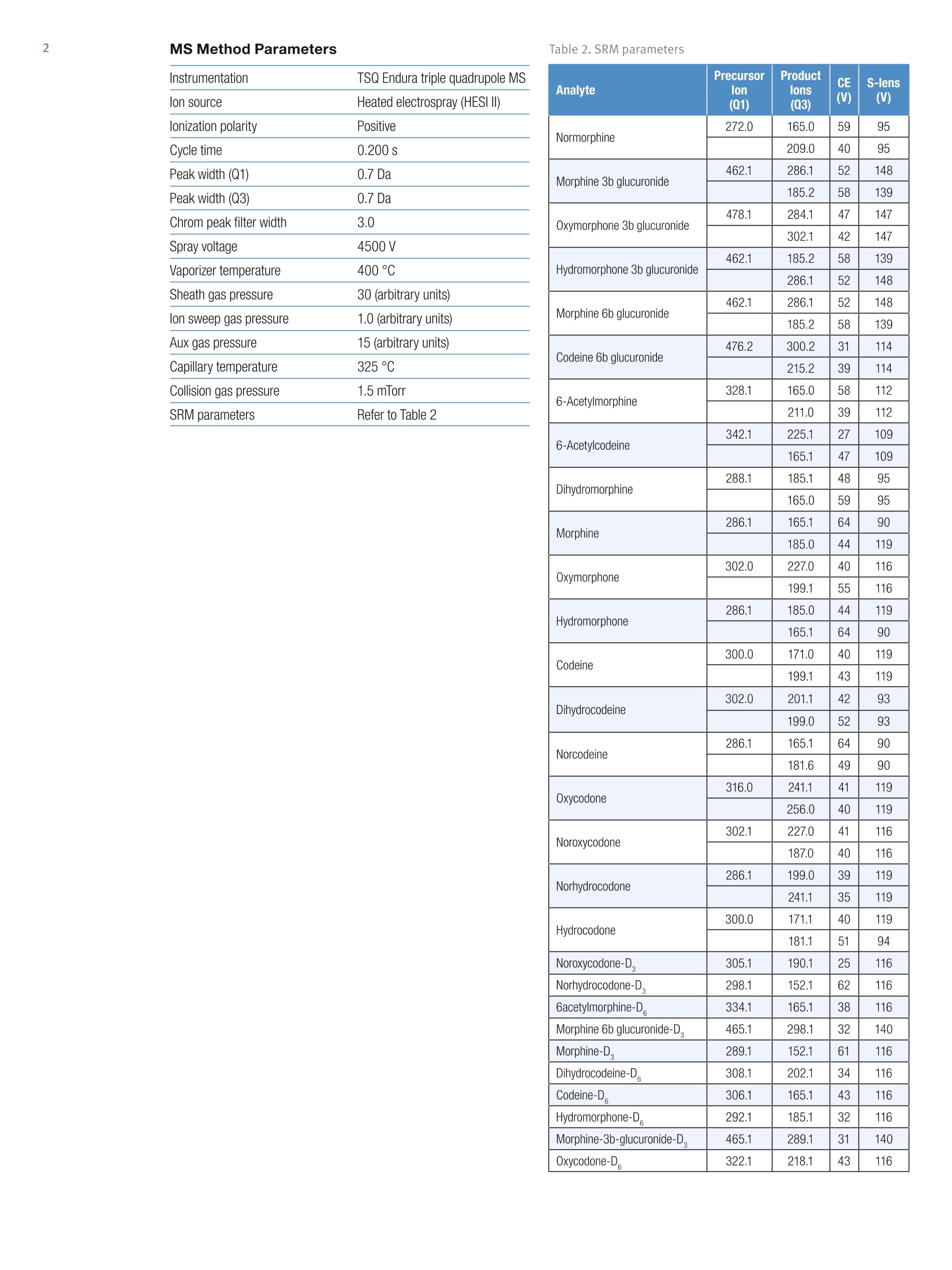
MS Method Parameters 3 Quantitative Forensic Analysis of Opiates,Opioids, and Their Metabolites in HumanUrine Without Hydrolysis Sarah Fair Wandland, Kerry Hassell, Joseph Herman, Thermo Fisher Scientific, Franklin, MA Introduction Analysis of opiate and opioid metabolites in urine is mostoften done with a hydrolysis step that make total samplepreparation time up to 24 hours. The method describedhere eliminates the hydrolysis step by analyzing theconjugated metabolites intact using a Thermo Scientific"Prelude SPLCsystem for sample preparation and aThermo Scientific" TSQ Endura triple quadrupolemass spectrometer for analysis. Experimental Sample Preparation Urine samples, which were free of opiates, were dilutedtwo-fold with water and methanol (95:5) containinginternal standards. There were a total of 10 deuteratedinternal standards in solution at a concentration of50 ng/mL. After the addition of the internal standards,50 pL of each sample were injected onto the analyticalcolumn at a temperature of 27 °C. Calibration standards containing all 19 compounds atconcentrations ranging from 5 to 500 ng/mL were preparedin urine. Quality control (QC) samples were also preparedin urine at three levels: 12, 225, and 400 ng/mL. SPLC Method Parameters Instrumentation Prelude SPLC system (Figure 1) Analytical column Thermo Scientific AccucoreaQ column (100x2.1 mm, 2.6 um particle size), catalog# 17326-102130 Mobile phase A 0.1% formic acid in water (Fisher Chemical brand) Mobile phase B 0.1% formic acid in methanol (Fisher Chemical brand) Gradient Refer to Table 1 Figure 1. Prelude SPLC system with TSQ Endura triple quadrupolemass spectrometer Table 1. Gradient details Step Start(min) Time(S) Flow(mL/min) Grad. %A %B 1 0.00 20 0.40 Step 100.0 0.0 2 0.33 5 0.40 Step 92.0 8.0 3 0.42 50 0.40 Step 92.0 8.0 4 1.25 5 0.40 Step 75.0 25.0 5 1.33 130 0.40 Ramp 65.0 35.0 6 3.50 45 0.40 Step 0.0 100.0 7 4.25 100 0.40 Step 100.0 0.0 Table 2. SRM parameters Instrumentation TSQ Endura triple quadrupole MS lon source Heated electrospray (HESI II) lonization polarity Positive Cycle time 0.200 s Peak width (Q1) 0.7 Da Peak width (Q3) 0.7 Da Chrom peak filter width 3.0 Spray voltage 4500V Vaporizer temperature 400°C Sheath gas pressure 30 (arbitrary units) Ion sweep gas pressure 1.0 (arbitrary units) Aux gas pressure 15 (arbitrary units) Capillary temperature 325°℃ Collision gas pressure 1.5 mTorr SRM parameters Refer to Table 2 Method Validation Accuracy and precision were tested by using five replicatesof three levels of quality controls over four days andquantitating them using calibration curves at thebeginning and end of the batchrun. The fourth day ofaccuracy and precision was performed in real urine tocross-verify the use of real matrix. Carryover wascalculated by dividing the total analyte signal of the lowerlimit of quantitation (LLOQ) by the total analyte signalfound in the matrix blank after the upper limit ofquantitation (ULOQ). This number could not exceed 20%of the total LLOQ signal. Additionally, autosamplerstability (24 hours at 4°C) was determined by runningQC samples that were refrigerated overnight in theautosampler to a new calibration curve the following day. Results and Discussion The assay precision had %RSD values that were within20.0% at the LLOQ and low QC, and within 15.0% forall other QC and calibration standard levels. Additionally,accuracy was within 20.0% at the LLOQ and low QC,and within 15% for all other QC and calibration standardlevels. All of these results are shown in Table 3. The short 4.25 minute analytical method provided ampleresolution for all isobaric compounds. All the analytespassed acceptance criteria for carryover, matrix effects,and autosampler stability. Example chromatograms foreach of the compounds are shown in Figure 2. Table 3. Accuracy and precision results Analyte Accuracy Precision (%RSD) Intra-Assay Inter-Assay Normorphine 94.6 <14.3 <5.7 Dihydromorphine 102 <14.1 <8.2 Morphine 99.2 <8.8 <4.8 Oxymorphone 103 <10.3 <3.5 Hydromorphone 102 <14.1 <5.8 Norcodeine 98.6 <9.6 <4.1 Dihydrocodeine 99.5 <11.1 <5.3 Codeine 99.2 <13.6 <5.7 Norhydrocodone 98.2 <13.5 <9.2 Oxycodone 99.4 <14.1 <5.8 Noroxycodone 100 <11.6 <10.4 Hydrocodone 95.2 <7.4 <5.0 6-Acetylmorphine 103 <9.7 <4.4 Codeine 6B glucuronide 102 <8.5 <4.1 Oxymorphone 3B glucuronide 100 <14.4 <4.4 Hydromorphone 3B glucuronide 108 <7.9 <5.7 Morphine 3B glucuronide 98.5 <14.9 <4.1 Morphine 6B glucuronide 99.0 <10.8 <3.7 6-Acetylcodeine 102 <6.1 <6.9 Conclusion A forensic method for analysis of opiates, opioids, andtheir metabolites without hydrolysis has been developedusing the Prelude SPLC system and TSQ Endura MS. Byeliminating the hydrolysis step, the sample preparationtime and analysis cost was drastically reduced. The LCmethod on the Prelude SPLC system/TSQ Endura MSprovided ample resolution for all isobaric compounds and www.thermoscientific.com ◎2014 Thermo Fisher Scientific Inc. All rights reserved. ISO is a registered trademark of the International Organization for Standardization(Organisation Internationale De Normalization). All other trademarks are the property of Thermo Fisher Scientific and its subsidiaries. Thisinformation is presented as an example of the capabilities of Thermo Fisher Scientific products. It is not intended to encourage use of theseproducts in any manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change.Not all products are available in all countries. Please consult your local sales representative for details. Africa +43 1 333 50 34 0 Japan +81 45 453 9100 Australia +61 3 9757 4300 Latin America +1 561 6888700 Austria +43 810 282 206 Middle East +431 333 50 340 Denmark +45 70 23 6260Europe-Other +43 1 333 5034 0Finland +358 9 3291 0200France +33 1 60 92 48 00Germany +49 6103 408 1014India +91 22 6742 9494Italy +39 02 950 591 SCIENTIFIC AThermo Fisher Scientific BrandANE
关闭-
1/4

-
2/4
还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
赛默飞质谱分析为您提供《人尿中毒品检测方案(液质联用仪)》,该方案主要用于人尿中毒品检测,参考标准《暂无》,《人尿中毒品检测方案(液质联用仪)》用到的仪器有赛默飞TSQ Endura三重四极杆质谱仪、赛默飞Prelude SPLC样品前处理及液相系统。
我要纠错
推荐专场
相关方案










 咨询
咨询






