方案详情文
智能文字提取功能测试中
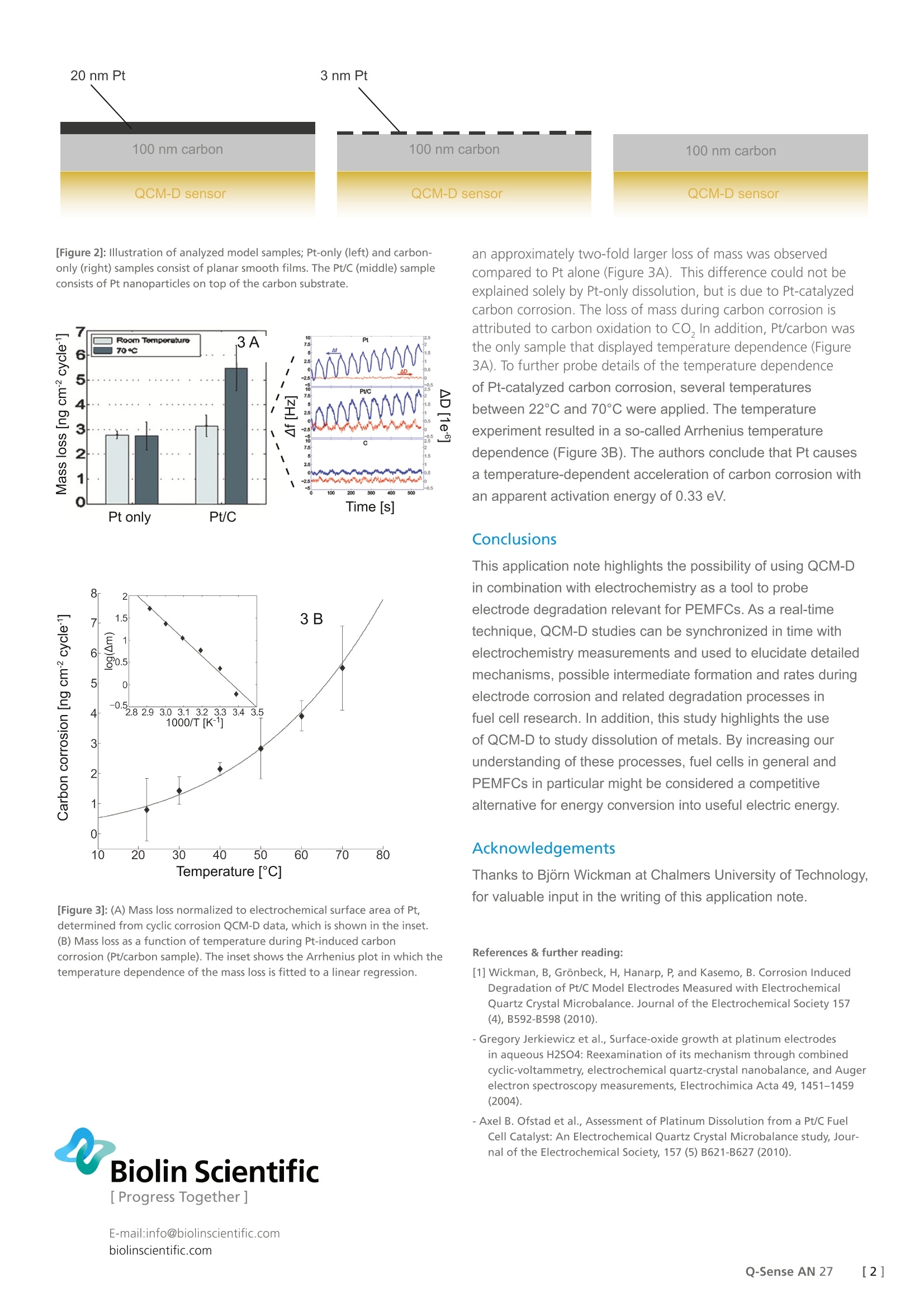
Biolin Scientific[ Progress Together] [Application Note] 27 Analyzing fuel cell electrode corrosion byelectrochemical QCM-D Polymer Electrolyte Membrane Fuel Cells (PEMFCs) are promising for use in appli-cations such as cars, laptops and stationary applications. However, corrosion of theelectrodes shortens their lifetime, and better understanding of these processes isnecessary to improve PEMFCs and make them a realistic alternative as a source ofelectricity. Here, QCM-D combined with electrochemistry was used to investigatecorrosion of PEMFC electrodes. PEMFCs use electrochemical reactions of oxygen and hydrogengas to convert chemical energy into electrical energy. Polymerelectrolytes have some obvious advantages in comparison to otherfuel cell technologies. The low operating temperature enables afast start-up, a requirement for most applications. However, thelow temperature also implies that a catalyst is needed for theelectrochemical reactions, normally platinum (Pt). In contrast toother possible electrolytes, a polymer membrane decreases thevolume necessary to provide the same amount of energy. The overall oxidation of hydrogen is divided into electrochemicalhalf-cell reactions that occur on the anode and cathode (Figure1). A typical PEMFC electrode consists of Pt nanoparticles finelydistributed onto a porous surface of carbon particles. Criticalparameters for the lifetime of these electrodes are corrosion ofcarbon support and Pt dissolution, leading to a loss of electrodesurface area. Consequently, the short lifetime of PEMFC has so farlimited their use as a competitive source for electrical energy. [Figure 1]: Schematic illustration of a typical Polymer Electrolyte MembraneFuel Cell (PEMFC). The anode and cathode are physically separated by amembrane structure (PEM). The analysis of corrosion-induced degradation of Pt/carbonelectrodes is normally monitored by indirect methods, greatlylimiting the information acquired. In this study, Quartz CrystalMicrobalance with Dissipation (QCM-D) monitoring combinedwith electrochemistry (EQCM-D) was used as a more direct wayof probing electrode degradation. The EQCM-D unveiled thetotal mass loss upon corrosion and also probed the temperaturedependence of these reactions in real time, demonstrating thatQCM-D can be used to further develop more stable fuel cells. Experimental Three differently coated QCM-D sensors were used to mimic theelectrodes used in PEMFCs (Figure 2). The coated sensors werePt-only, carbon-only and Pt/carbon on which Pt partially coversthe carbon nanoparticle surface. These sensors were subjectedto cyclic corrosion EQCM-D measurements with synchronizedtime scales in a three-electrode flow-cell setup. Measurementswere made at room temperature and up to 70 C, to probe massloss as an indicator of electrode corrosion (Afand AD responsesto the cyclic corrosion at 70°℃ are shown in the inset in Fig. 3A). The lack of change in dissipation (△D) justified calculatingthe mass loss with the Sauerbrey equation, using the change infrequency (Af) from the third and fifth overtones in the QCM-Dmeasurements. Results and discussion The three sensor surfaces responded differently to the cycliccorrosion experiments, as revealed by QCM-D measurements. Itwas noted that the carbon-only sensor displayed very marginalmass losses (Figure 3A inset) and the higher mass loss during thePt-only experiment is due to Pt dissolution. The values obtainedcorrespond well with previous studies. For the Pt/carbon sample, [Figure 2]: Illustration of analyzed model samples; Pt-only (left) and carbon-only (right) samples consist of planar smooth films. The Pt/C (middle) sampleconsists of Pt nanoparticles on top of the carbon substrate. Temperature [C] [Figure 3]: (A) Mass loss normalized to electrochemical surface area of Pt,determined from cyclic corrosion QCM-D data, which is shown in the inset.(B) Mass loss as a function of temperature during Pt-induced carboncorrosion (Pt/carbon sample). The inset shows the Arrhenius plot in which thetemperature dependence of the mass loss is fitted to a linear regression. Biolin Scientific an approximately two-fold larger loss of mass was observedcompared to Pt alone (Figure 3A). This difference could not beexplained solely by Pt-only dissolution, but is due to Pt-catalyzedcarbon corrosion. The loss of mass during carbon corrosion isattributed to carbon oxidation to CO, In addition, Pt/carbon wasthe only sample that displayed temperature dependence (Figure3A). To further probe details of the temperature dependenceof Pt-catalyzed carbon corrosion, several temperaturesbetween 22°℃ and 70℃ were applied. The temperatureexperiment resulted in a so-called Arrhenius temperaturedependence (Figure 3B). The authors conclude that Pt causesa temperature-dependent acceleration of carbon corrosion withan apparent activation energy of 0.33 eV. Conclusions This application note highlights the possibility of using QCM-Din combination with electrochemistry as a tool to probeelectrode degradation relevant for PEMFCs. As a real-timetechnique, QCM-D studies can be synchronized in time withelectrochemistry measurements and used to elucidate detailedmechanisms, possible intermediate formation and rates duringelectrode corrosion and related degradation processes infuel cell research.In addition, this study highlights the useof QCM-D to study dissolution of metals. By increasing ourunderstanding of these processes, fuel cells in general andPEMFCs in particular might be considered a competitivealternative for energy conversion into useful electric energy. Acknowledgements Thanks to Bjorn Wickman at Chalmers University of Technology,for valuable input in the writing of this application note. References & further reading: ( [1] Wickman, B, Gronbeck, H, Hanarp, P, and Kasemo, B. Corrosion Induced Degradation of Pt/C M odel Electrodes M e asured wi t h Electrochemical Quartz Crystal Microbalance. Journal of the Electrochemical Society 157 (4), B592-B598 ( 201 0 ). ) ( - Gr eg or y Jerkiewicz et al . , Surface-oxide g rowth at pl atinum electrodes in aqueous H2SO4: Reexamination of its mechanism through c ombined cy clic-voltammetry , e lectrochemic a l q uar tz-crystal n anobalance, and Au g er electron spectrosc opy measurements, E l ectrochimic a Acta 49, 1 451-14 5 9 (2004). ) -Axel B. Ofstad et al., Assessment of Platinum Dissolution from a Pt/C FuelCell Catalyst: An Electrochemical Quartz Crystal Microbalance study, Jour-nal of the Electrochemical Society, 157 (5) B621-B627 (2010). []Q-Sense AN
关闭-
1/2

-
2/2
产品配置单
瑞典百欧林科技有限公司为您提供《通过QCM-D电化学模块研究燃料电池电极腐蚀情况》,该方案主要用于燃料电池中null检测,参考标准《暂无》,《通过QCM-D电化学模块研究燃料电池电极腐蚀情况》用到的仪器有QSense卓越版四通道石英晶体微天平、QSense Explorer扩展版石英晶体微天平、QSense全自动八通道石英晶体微天平。
我要纠错
相关方案




 咨询
咨询





