方案详情文
智能文字提取功能测试中
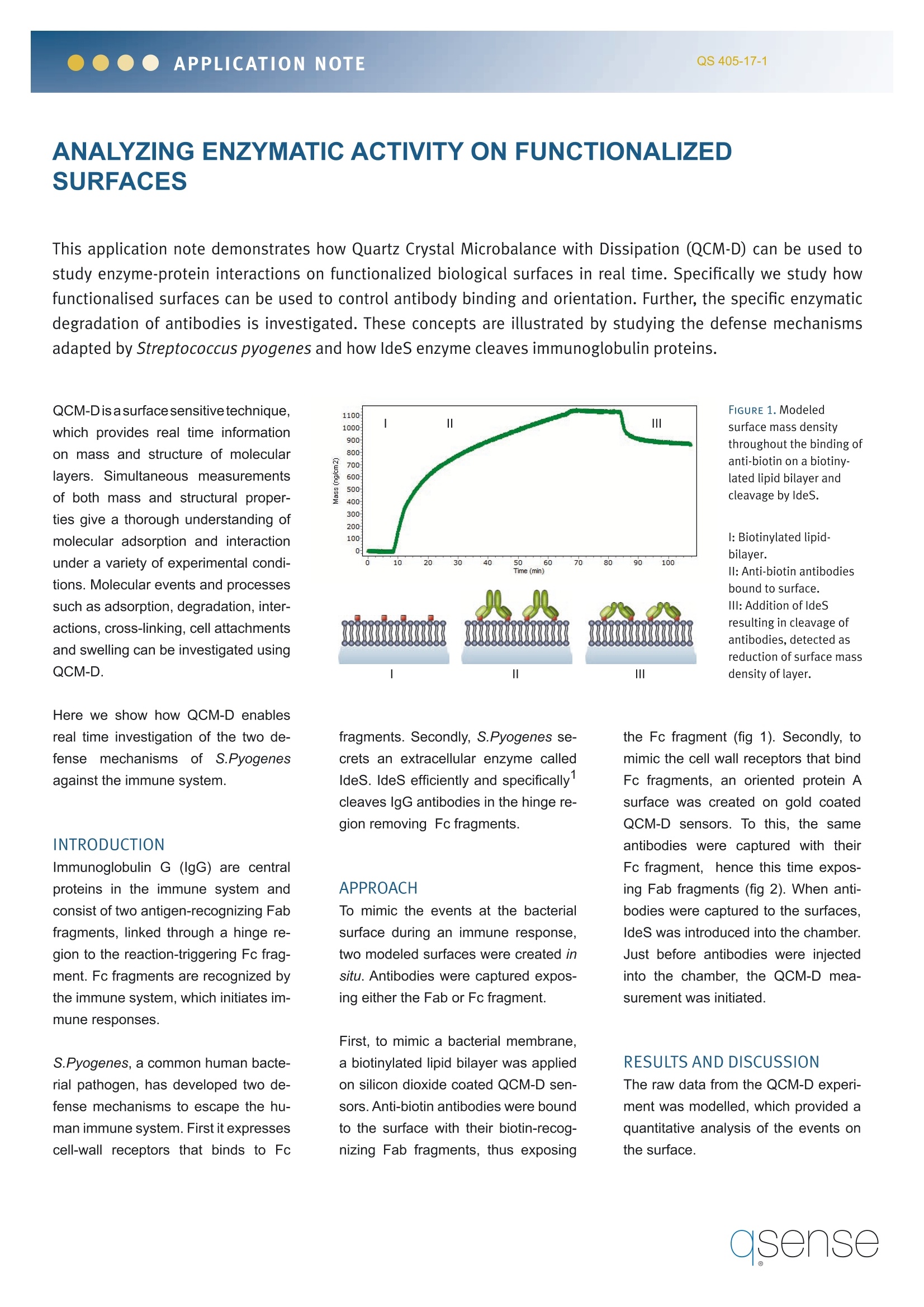
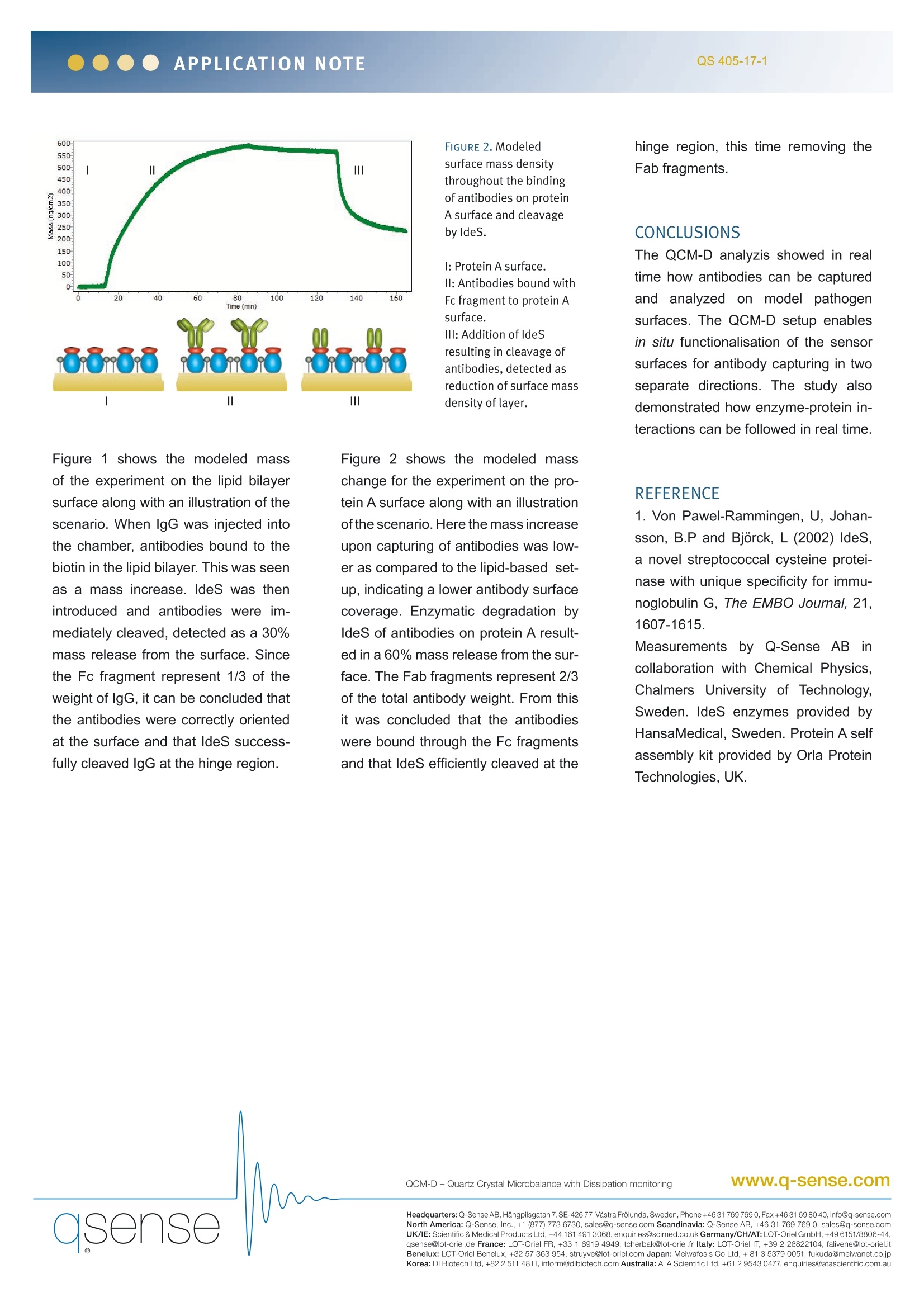
APPLICATION NOTEQS 405-17-1 ANALYZING ENZYMATIC ACTIVITY ON FUNCTIONALIZEDSURFACES This application note demonstrates how Quartz Crystal Microbalance with Dissipation (QCM-D) can be used tostudy enzyme-protein interactions on functionalized biological surfaces in real time. Specifically we study howfunctionalised surfaces can be used to control antibody binding and orientation. Further,the specific enzymaticdegradation of antibodies is investigated. These concepts are illustrated by studying the defense mechanismsadapted by Streptococcus pyogenes and how IdeS enzyme cleaves immunoglobulin proteins. QCM-Disasurfacesensitivetechnique,which provides real time informationon mass and structure of molecularlayers. Simultaneous measurementsof both mass and structural proper-ties give a thorough understanding ofmolecular adsorption and interactionunder a variety of experimental condi-tions. Molecular events and processessuch as adsorption, degradation, inter-actions, cross-linking, cell attachmentsand swelling can be investigated usingQCM-D. Here we show how QCM-D enablesreal time investigation of the two de-fense mechanisms of S.Pyogenesagainst the immune system. INTRODUCTION Immunoglobulin G (lgG) are centralproteins in the immune system andconsist of two antigen-recognizing Fabfragments, linked through a hinge re-gion to the reaction-triggering Fc frag-ment. Fc fragments are recognized bythe immune system, which initiates im-mune responses. S.Pyogenes, a common human bacte-rial pathogen, has developed two de-fense mechanisms to escape the hu-man immune system. First it expressescell-wall receptors that binds to Fc fragments. Secondly, S.Pyogenes se-crets an extracellular enzyme calledldeS. IdeS efficiently and specifically'cleaves IgG antibodies in the hinge re-gion removing Fc fragments. APPROACH To mimic the events at the bacterialsurface during an immune response,two modeled surfaces were created insitu. Antibodies were captured expos-ing either the Fab or Fc fragment. First, to mimic a bacterial membrane,a biotinylated lipid bilayer was appliedon silicon dioxide coated QCM-D sen-sors. Anti-biotin antibodies were boundto the surface with their biotin-recog-nizing Fab fragments, thus exposing the Fc fragment (fig 1).Secondly, tomimic the cell wall receptors that bindFc fragments, an oriented protein Asurface was created on gold coatedQCM-D sensors. To this, the sameantibodies were captured with theirFc fragment, hence this time expos-ing Fab fragments (fig 2). When anti-bodies were captured to the surfaces,IdeS was introduced into the chamber.Just before antibodies were injectedinto the chamber, the QCM-D mea-surement was initiated. RESULTS AND DISCUSSION The raw data from the QCM-D experi-ment was modelled, which provided aquantitative analysis of the events onthe surface. Figure 1 shows the modeled massof the experiment on the lipid bilayersurface along with an illustration of thescenario. When IgG was injected intothe chamber, antibodies bound to thebiotin in the lipid bilayer. This was seenas a mass increase. IdeS was thenintroduced and antibodies were im-mediately cleaved, detected as a 30%mass release from the surface. Sincethe Fc fragment represent 1/3 of theweight of lgG, it can be concluded thatthe antibodies were correctly orientedat the surface and that IdeS success-fully cleaved lgG at the hinge region. Figure 2 shows the modeled masschange for the experiment on the pro-tein A surface along with an illustrationof the scenario. Here the mass increaseupon capturing of antibodies was low-er as compared to the lipid-based set-up, indicating a lower antibody surfacecoverage. Enzymatic degradation byldeS of antibodies on protein A result-ed in a 60% mass release from thesur-face. The Fab fragments represent 2/3of the total antibody weight. From thisit was concluded that the antibodieswere bound through the Fc fragmentsand that IdeS efficiently cleaved at the hinge region, this time removing theFab fragments. CONCLUSIONS The QCM-D analyzis showed in realtime how antibodies can be capturedand analyzed on model pathogensurfaces. The QCM-D setup enablesin situ functionalisation of the sensorsurfaces for antibody capturing in twoseparate directions. The study alsodemonstrated how enzyme-protein in-teractions can be followed in real time. REFERENCE 1. Von Pawel-Rammingen, U, Johan-sson, B.P and Bjorck, L (2002) IdeS,a novel streptococcal cysteine protei-nase with unique specificity for immu-noglobulin G, The EMBO Journal, 21,1607-1615. Measurements by Q-Sense AB incollaboration with Chemical Physics,Chalmers University of Technology,Sweden. IdeS enzymes provided byHansaMedical, Sweden. Protein A selfassembly kit provided by Orla ProteinTechnologies, UK. ( Headquarters: Q-Sense AB, Ha ngpil sg atan 7, SE - 4 2 6 77 Vast r a F r o lu n da,Sweden, Ph one+ 46 31 7 69 7690 , F a x+ 46 31 69 80 4 0 , i nfo@q - sense. c om North America: Q-Sense, I n c . , +1 (877) 773 67 3 0, s a l e s @q -s en s e. c om Scandinavia: Q -S e ns e AB, + 46 3 1 769 769 0,sa l e s @q- sen se.c o mUK/IE:Scie ntifi c & Medical Pr o duc t s L td, +4 4 1 6 1 49130 6 8, en q u i ries@s ci med.co.uk Germany/CH/AT:LOT-Orie l GmbH, + 4 9 6 151/88 06 -44, ) asense qsense@lot-oriel.de France: LOT-Oriel FR, + tcherbak@lot-oriel.fr Italy: LOT-Oriel IT, +falivene@lot-oriel.itBenelux: LOT-Oriel Benelux, +struyve@lot-oriel.com Japan: Meiwafosis Co Ltd, + fukuda@meiwanet.co.jrKorea: DI Biotech Ltd, + inform@dibiotech.com Australia: ATA Scientific Ltd,+ enquiries@atascientific.com.au
关闭-
1/2
-
2/2
产品配置单
瑞典百欧林科技有限公司为您提供《使用QCM-D功能性芯片检测酶活性》,该方案主要用于其他中null检测,参考标准《暂无》,《使用QCM-D功能性芯片检测酶活性》用到的仪器有QSense卓越版四通道石英晶体微天平、QSense Explorer扩展版石英晶体微天平、QSense全自动八通道石英晶体微天平。
我要纠错
相关方案





 咨询
咨询