方案详情文
智能文字提取功能测试中
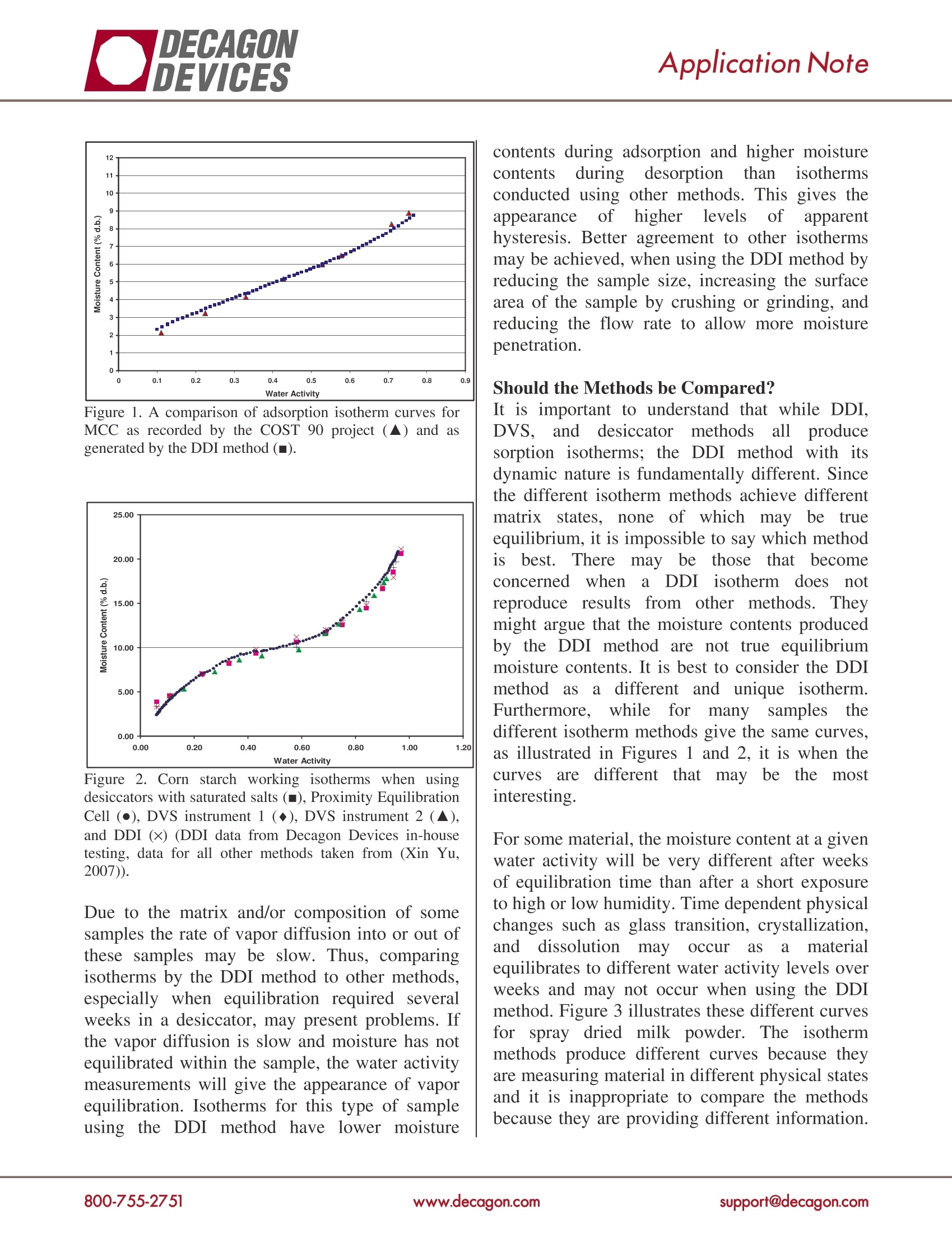
Application Note Dynamic Dewpoint Isotherm verses Other Moisture Sorption Isotherm MethodsBy Brady Carter and Anthony Fontana Ph.D. Moisture Sorption Isotherm Methods The Dynamic Dewpoint Isotherm (DDI) methodused by the AquaSorp Isotherm Generator is likeother moisture sorption isotherm methods in thatit provides the relationship between water activityThe Dynamic Dewpoint Isotherm Methodand moisture content. However, the DDI methodThe DDI method is very different from the otheris unparalleled in the detail and speed with which isotherm methods as neither water activity norit produces isotherm curves and the amount of moisture content is controlled. Rather wateradditional information not previously possible activity is directly measured using a standardwith other methods. chilled mirror dewpoint sensor and moisturecontent is gravimetrically tracked using a balance.Wetting is imposed by saturating the air withTraditionalisotherm methodsdepend on water before it enters the chamber and drying isestablishingthe equilibration of samplesto achieved by passing air through desiccant beforeknown water activity values and then measuring it enters the sample chamber. The method isthe moisture contents of these samples. Common dynamic because the sample is not required toto all these isotherm methods is the dependence equilibrate to a known water activity level; ratheron equilibration to known water activity levels to its water activity is directly measured at eachdetermine each data point’s water activity. Since point. The DDI method without these longtrue equilibration between the sample and the equilibration periods dramatically reduces thevapor source requires an infinitely long period of time required to develop a moisture sorptiontime, an apparent equilibrium when the weight curve with an unmatched amount of data points.stops changing by an acceptable level is used. In addition, only water and desiccant are requiredWidening the tolerance in weight change will to run an isotherm. Currently, the AquaSorpspeed up the isotherm process but calls into Isotherm Generator is the only instrument thatquestion the validity of the water activity values. utilizes the DDI method.The static desiccator method is performed byplacingsamples in sealed chambers ovveer For most samples, especially those with fastsaturated salt slurries. Different water activity vapor diffusion, penetration by water vapor intolevels are achieved by using different saturated the whole sample is rapid and isotherms from thesalts. Instrumentation, knownas controlled DDI method are comparable to tthe otheratmosphere microbalances (CAM)exists to methods. Figure 1 compares the adsorptioncontinuously monitor weight changes and control isotherm for microcrystalline cellulose (MCC)relative humidity by adjusting a mixture of wet from a traditional static desiccator method fromand dry gas streams. Different relative humidity the COST-90 project (Wolf et al., 1985; Jowittlevels are achieved by changing the ratio of dry to and Wagstaffe, 1989) to the DDI method. Figurewet gas. Some instruments are programmed to 2 compares the isotherm curves for corn starchautomatically change the water activity in a from traditional, DVS, and DDIisothermdynamic stepwise progression, usually referred to methods. As shown in both Figures 1 and 2, theas Dynamic Vapor Sorption (DVS). The sample DDI method has very good agreement with theis held at each relative humidity level until weight other moisture sorption isotherm methods. stops changing before moving to the next relativehumidity level. Comparing the Methods Figure 1. A comparison of adsorption isotherm curves forMCC as recorded by the COST 90 project (▲) and asgenerated by the DDI method (). Figure 2. Corn starch working isotherms when usingdesiccators with saturated salts (), Proximity EquilibrationCell (), DVS instrument 1 (◆), DVS instrument 2 (),and DDI (×) (DDI data from Decagon Devices in-housetesting, data for all other methods taken from (Xin Yu,2007)). Due to the matrix and/or composition of somesamples the rate of vapor diffusion into or out ofthese samples may be slow. Thus, comparingisotherms by the DDI method to other methods,especially when equilibration required severalweeks in a desiccator, may present problems. Ifthe vapor diffusion is slow and moisture has notequilibrated within the sample, the water activitymeasurements will give the appearance of vaporequilibration. Isotherms for this type of sampleusing the DDI method have lower moisture contents during adsorption and higher moisturecontents during desorption than isothermsconducted using other methods. This gives theippearance of higher levels ofapparenthysteresis. Better agreement to other isothermsmay be achieved, when using the DDI method byreducing the sample size, increasing the surfacearea of the sample by crushing or grinding, andreducing the flow rate to allow more moisturepenetration. Should the Methods be Compared? It is important to understand that while DDI,DVS, and desiccator methods all producesorption isotherms; the DDI method with itsdynamic nature is fundamentally different. Sincethe different isotherm methods achieve differentmatrix states, none of which may be trueequilibrium, it is impossible to say which method1Sbest. There maybe those that becomeconcerned when a DDI isotherm does notreproduce results from other methods. Theymight argue that the moisture contents producedby the DDI method are not true equilibriummoisture contents. It is best to consider the DDIInethod as a different and unique isotherm.Furthermore, while for many samples thedifferent isotherm methods give the same curves,as illustrated in Figures 1 and 2, it is when thecurVesare different that may be the iinteresting. For some material, the moisture content at a givenwater activity will be very different after weeksof equilibration time than after a short exposureto high or low humidity. Time dependent physicalchanges such as glass transition, crystallization,and dissolution mayoccur as a materialequilibrates to different water activity levels overweeks and may not occur when using the DDImethod. Figure 3 illustrates these different curvesfor spray dried milk powder. The isothermmethods produce different curves because theyare measuring material in different physical statesand it is inappropriate to compare the methodsbecause they are providing different information. This does not mean that the DDI method is wrongand the other methods; are right. In fact, thedynamic nature of the DDI method may actuallyillustrate a more accurate sorption characteristicof this type of material in real conditions sincesamples are rarely exposed to changes in moisturein stepwise progression but instead in a dynamicprogression. Figure 3. A comparison of adsorption isotherms for spraydried milk powder conducted using traditional desiccatormethod and fitted to the GAB equation(■)and the DDImethod (no model fitting)(▲). The Value of the DDI Method The dynamic nature of the DDI method allowsfor the rapid (1-2 days) generation of a large dataset not practically possible with other isothermmethods as it would take an inhibitory length oftime. Typically, the DDI isotherm data set of 50to 70 points has a 0.01aw change between eachcollected data point. This detailed data resolutioneliminates the need for extrapolationorinterpolation, and gives a direct detailed view ofthe sorption curve. Figure 3 illustrates isothermsfor spray dried milk powder produced using thedesiccator method and the DDI method. Time andlabor constraints limit the number of data pointsgenerated by the desiccator method and requiresinterpolation using the GAB equation to completethe curve. The detail data resolution of the DDImethodrproducesat completeeCcurve makinginterpolation by a model unnecessary. In fact,using a smooth curve model to characterize the isotherm would miss the most import results ofthe DDI analysis. The DDI data directly showsthat important matrix changes are occurring asillustrated by changes in sorption rates, at 0.420,0.559, and 0.724 aw. Phasetransitions such as glass transitions,crystallization, and dissolution are identifiable bythe DDI method. It would be impossible toidentify any of the phase changes using just thedesiccator method data as fitted to the GABequation in Figure 3. This explains why manybelieve that moisture sorption isotherm data couldnot be used to identify phase transitions pointssuchasglass transition. However, phasetransitions are identifiable by the DDI method. The Dynamic Dewpoint Isotherm Method is aunique analysis method that provides importantinformation not previously possible with otherisotherm methods. The DDI method with itsunparalleled detail, speed, and amount ofinformation generated from each isotherm curvewill greatly aid in the understanding of waterinteractions within materials. References ( Jowitt, R. and Wagstaffe, P. J. The certification of thewater content of microcrystalline cellulose (MCC) at 10 v water activiti e s - CRM 302. 1 989.Luxembourg, Office fo r Of f icial P u blications ofthe European Communities. pp. 1-54. ) Wolf,W.,W.E.L.Spiess, and G.Jung. 1985.Standardization of Isotherm Measurements (cost-project 90 and 90 BIS). p. 661-679. In D.Simatos,and J.L.Multon (ed.) Properties of Water in Foodsin Relation to Quality and Stability. MartinusNijhoff, Boston. Xin Yu. 2007. Investigation of moisture sorptionproperties of food materials using saturated saltsolution and humidity generating techniques. Ph.D.thesis, University of Illinois at Urbana-Champaign. Decagon Devices2365 NE Hopkins CourtPullman Washington 99163 ( C 2 008 13506-00 Decagon Devices, Printed in USA ) upport@decagon.comwww.decagon.com
关闭-
1/3

-
2/3
还剩1页未读,是否继续阅读?
继续免费阅读全文产品配置单
METER Group, Inc.北京办事处为您提供《食品中水分吸附等温线检测方案(蒸汽吸附仪)》,该方案主要用于其他食品中水分吸附等温线检测,参考标准《暂无》,《食品中水分吸附等温线检测方案(蒸汽吸附仪)》用到的仪器有Aqualab VSA水分吸附分析仪。
我要纠错
推荐专场
相关方案






 咨询
咨询




