方案详情文
智能文字提取功能测试中
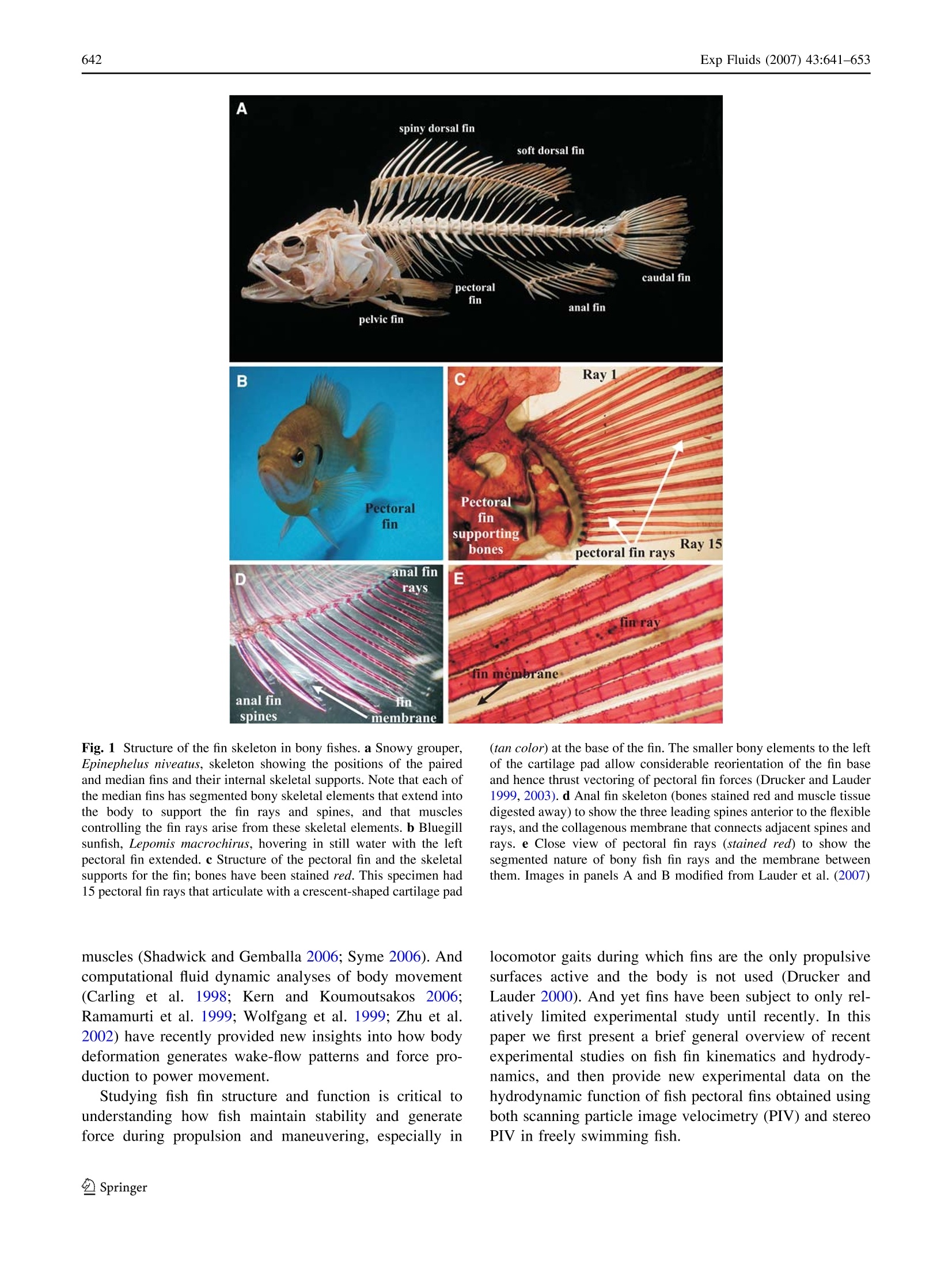
Exp Fluids (2007) 43:641-653DOI 10.1007/s00348-007-0357-4 Exp Fluids (2007) 43:641-653642 Fish locomotion: kinematics and hydrodynamicsof flexible foil-like fins George V. Lauder· Peter G. A. Madden Abstract1TThe fins of fishes are remarkable propulsivedevices that appear at the origin of fishes about 500 millionyears ago and have been a key feature of fish evolutionarydiversification. Most fish speciesSpossess both mediar(midline) dorsal, anal, and caudal fins as well as pairedpectoral and pelvic fins. Fish fins are supported by jointedskeletal elements, fin rays, that in turn support a thin col-lagenous membrane.Muscles at the base of the fin attach toand actuate each fin ray, and fish fins thus generate theirown hydrodynamic wake during locomotion, in addition tofluid motion induced by undulation of the body. In bonyfishes, the jointed fin rays can be actively deformed and thefin surface can thus actively resist hydrodynamic loading.Fish fins are highly flexible, exhibit considerable defor-mation during locomotion, and can interact hydrodynami-cally during both propulsion andmaneuvering. Forexample, the dorsal and anal fins shed a vortex wake thatgreatly modifies the flow environment experienced by thetail fin. New experimental kinematic and hydrodynamicdata are presented for pectoral fin function in bluegillsunfish. The highly flexible sunfish pectoral fin moves in acomplex manner with two leading edges, a spanwise waveof bending, and substantial changes in area through the finbeat cycle. Data from scanning particle image velocimetry(PIV) and time-resolved stereo PIV show that the pectoralfin generates thrust throughout the fin beat cycle, and thatthere is no time of net drag. Continuous thrust production isdue to fin flexibility which enables some part of the fin togenerate thrust at all times and to smooth out oscillations ( G.V. L auder ()· P. G. A. Madden Museum of Comparative Zoology,Harvard U niversity, 26 Oxford S treet, Cambridge, MA 02138, USAe-mail: glauder@oeb.harvard.edu ) that might arise at the transition from outstroke to instrokeduring the movement cycle. Computational fluid dynamicanalyses of sunfish pectoral fin function corroborate thisconclusion. Future research on fish fin function will benefitconsiderably from close integration with studies of roboticmodel fins. 1 Introduction Of the most prominent characteristics of early fossil fishesare the median and paired fins that project from the bodysurface into the surrounding fluid. Fins have been a fun-damental design feature of fishes since their origin ~500million years ago, predating even the origin of jaws and theability of early fishes to bite and chew food. The sub-sequent evolutionary diversification of fishes into over28,000 species has been marked by differentiation of dis-tinct midline and paired fin structures and the evolution ofcomplex skeletal supports and control musculature forthese fins (Lauder 2006). Most fishes possess median(midline) dorsal, anal, and caudal (tail) fins, as well aspaired pectoral and pelvic fins (Fig.1) for a total of sevendiscrete control surfaces in addition to the body surface.Fish fins play a prominent role in the control of body po-sition and stability and in generating locomotor forcesduring propulsion and maneuvering. But the vast majorityof the work to date on fish propulsion has focused on thebody surface. Patterns of body deformation during loco-motion and their hydrodynamic effect have been well de-scribed (reviewed in Fish and Lauder 2006; Lauder andTytell 2006), as has the arrangement of body musculatureand the activity and contractile characteristics of these B Ray1 PectoralPectoralfinfinsupportingRay 15bonespectoral fin raysDanal finEraysfin rayfin membraneanal fin fin spines membrane Fig. 1 Structure of the fin skeleton in bony fishes. a Snowy grouper,Epinephelus niveatus, skeleton showing the positions of the pairedand median fins and their internal skeletal supports. Note that each ofthe median fins has segmented bony skeletal elements that extend intothe body to support the fin rays and spines, and that musclescontrolling the fin rays arise from these skeletal elements. b Bluegillsunfish, Lepomis macrochirus, hovering in still water with the leftpectoral fin extended.c Structure of the pectoral fin and the skeletalsupports for the fin; bones have been stained red. This specimen had15 pectoral fin rays that articulate with a crescent-shaped cartilage pad muscles (Shadwick and Gemballa 2006; Syme 2006). Andcomputational fluid dynamic analyses of body movement(Carling et al. 1998;Kern and Koumoutsakos 22006:Ramamurti et al. 1999; Wolfgang et al. 1999; Zhu et al.2002) have recently provided new insights into how bodydeformation generates wake-flow patterns and force pro-duction to power movement. Studying fish fin structure and function is critical tounderstanding how fish maintain stability and generateforce during propulsion and maneuvering, especially in (tan color) at the base of the fin. The smaller bony elements to the leftof the cartilage pad allow considerable reorientation of the fin baseand hence thrust vectoring of pectoral fin forces (Drucker and Lauder1999, 2003).d Anal fin skeleton (bones stained red and muscle tissuedigested away) to show the three leading spines anterior to the flexiblerays, and the collagenous membrane that connects adjacent spines andrays. e Close view of pectoral fin rays (stained red) to show thesegmented nature of bony fish fin rays and the membrane betweenthem. Images in panels A and B modified from Lauder et al. (2007) locomotor gaits during which fins are the only propulsivesurfaces active and the body is not used (Drucker andLauder 2000). And yet fins have been subject to only rel-atively limited experimental study until recently. In thispaper we first present a brief general overview of recentexperimental studies on fish fin kinematics and hydrody-namics, and then provide new experimental data on thehydrodynamic function of fish pectoral fins obtained usingboth scanning particle image velocimetry (PIV) and stereoPIV in freely swimming fish. 2 Overview of fish fin structure and function Fish fins are supported by flexible bony or cartilaginous finrays that extend from the fin base into the fin surface andprovide support for the thin collagenous membrane thatconnects adjacent fin rays (Figs. 1, 2). Fin rays articulatewith the fin skeleton located inside the body wall whichsupports musculature that allows the fin rays to be activelymoved from side to side and elevated and depressed(Fig. 1, Geerlink and Videler 1974; Geerlink 1979; Jayneet al. 1996; Winterbottom 1974). Many fish also havedorsal and anal fins which have leading spiny portions ofthe fin (Fig. 1, Drucker and Lauder 2001b), and fin spinestypically can only be elevated and depressed; they possesslimited sideways mobility. The posterior region of thedorsal and anal fins is known as the“soft"dorsal or analfin and is supported only by flexible fin rays. Recordingsfrom fin musculature, which is distinct from the bodymuscles, unequivocally show that fins are actively movedduring swimming, and that this active movement generatesa vortex wake that passes downstream toward the tail,which thus intercepts the flow that is greatly altered fromthe free-stream (Drucker and Lauder 2001b, 2005; Jayneet al. 1996; Standen and Lauder 2007) (also see Fig. 4).The modulus of elasticity of bony fin rays is about 1 GPa,while the membrane in between fin rays has a modulus ofabout 0.3-1.0 MPa (Lauder et al. 2006). Fig. 2 Pectoral fin structure in bluegill sunfish, Lepomis macrochi-rus. a Schematic view of the pectoral fin which typically has 12-15fin rays. b Cross-section through fin rays at the level of the blue planeshown in panel A obtained with uCT scanning (see Alben et al. 2007)in which bone is whitish color, and fin collagen and membrane aregray. Cross-sectional image of rays (top) and close view of twoadjacent rays (below). Each fin ray is bilaminar, with two curved halfrays termed hemitrichs. c Schematic of the mechanical design of the The hallmark of fish fin functional design is the bendingof the fin rays which permits considerable flexibility of thepropulsive surface. The fin rays of the large fish grouptermed ray-finned fishes (but not sharks),,possessaremarkable bilaminar structure and muscular control thatallows fish to actively control fin surface conformation andcamber during locomotion. As illustrated in Fig. 2, eachbony fin ray is composed of two halves (termed hemitrichs)which are connected along their length by short collagenfibers and may be attached at the end of the ray (Albenet al. 2007; Geerlink and Videler 1987; Lauder 2006). Eachfin ray is actuated by four separate muscles, and thus asingle fin such as the pectoral fin of a bluegill sunfish(Lepomis macrochirus), which has about 14 fin rays,potentially has over 50 separate actuators that allow the finto be reoriented in three dimensions with control over theposition of each ray. Neural control of fin ray motion hasyet to be studied in detail, and the extent to which ana-tomically homologous muscles on neighboring fin rays canbe controlled independently is unknown. Most importantly,displacement of the two ray halves through the contractionof fin ray musculature at the base of the fin causes the finray to curve. Fish can thus actively alter the conformationof their propulsive surface by actively bending fin rays, andcan resist hydrodynamic loading, a phenomenon that isobserved most clearly during maneuvering (Fig. 2d). Oneresult of the complex control and bilaminar fin ray design bilaminar fin ray in bony fishes. Each fin ray has expanded bonyprocesses at the base of each hemitrich to which muscles attach (bluearrows). Differential actuation of fin ray muscles (red arrows) resultsin curvature of the fin ray. Fish can thus actively control the curvatureof their fin surface. d Frame from high-speed video of a bluegillsunfish during a turning maneuver, showing the fin surface (outlinedin yellow) curving into oncoming flow in fish fins is that, as illustrated in Sect. 5, fins can undergorather complex three-dimensional changes in shape duringlocomotion. 3 Methodology for experimental analyses of fishlocomotion Although experimental kinematic and hydrodynamic stud-ies of fishes swimming in large bodies of water and undernatural settings would be ideal, recent progress in under-standing the functional design of fishes has relied heavily oninducing locomotion in laboratory flow tanks, which permitprecise speed control and the induction of replicatedmaneuvering stimuli. Such studies have allowed bothdetailed kinematic studies of fin function and experimentalhydrodynamic recordings of wake flow patterns resultingfrom fin and body movement using PIV (e.g., Anderson1996; Drucker and Lauder 1999,2002a;Lauder andDrucker 2002, 2004;Liao et al. 2003; NauennandLauder 2002a, b; Wilga and Lauder 2002; Wolfgang et al.1999). Two critical enabling technologies that have beenresponsible for considerable progress in understanding fishlocomotor function in recent years are (1) the use of high-resolution (at least megapixel) high-speed video camerasacquiring images between 200 and 1000 frames per secondor more, and (2) the use of time-resolved PIV (Lauder andMadden 2008). Often these two techniques are used inconjunction with other approaches suchi as electricalrecordings of fin and body muscle activity patterns andmeasurement of muscle strain (e.g., Lauder et al. 2006), orthe use of biorobotic fish-like devices that enable precisecontrol of kinematics and exploration of broad (and evennon-biological) parameter spaces (Lauder et al. 2007). Therapid development of high-speed digital video technologyover the past decade coupled with the availability of lowercost continuous wave lasers has also permitted their use inPIV studies by biologists. This has allowed time-resolvedPIV (typically at 200-1,000 Hz) recordings of fin and bodywake flows with a temporal resolution 10-50 times that ofthe fin beat frequency, giving a detailed picture of vorticityproduction and the generation of biological flows near thebody and fins (Drucker and Lauder 1999, 2002a, 2003;Lauder 2000; Lauder and Drucker 2004). Megapixel high-speed video cameras have made the motion of individualfin rays visible (see Fig. 5, for example) and have per-mitted the accurate quantification of fin surface confor-mation (especially the bending of individual fin rays)which is critical to interpreting the kinematic causes ofwake flow patterns. One critical issue in experimental fluid dynamic studiesof freely swimming fishes is that the position of the fish in the laser light sheet used for PIV must be known. Wakeflow patterns produced by swimming fishes are highlysensitive to the location of the PIV light sheet, since thethree-dimensional structure of the wake changes substan-tially with height on the body, and wake flows from dorsaland anal fins change the flow structure substantially aboveand below the tail (Standen and Lauder 2007; Tytell 2006).Thus, we highly recommend the use of multiple high-speedcameras to image simultaneously fin kinematics, wake flowpatterns, and body position in the laser light sheet.Figure 3a shows an image of a brook trout swimming in arecirculating flow tank with dual light sheets generated bytwo argon-ion continuous wave lasers to study dorsal andanal fin function. Simultaneous use of multiple orthogonalhigh-speed video cameras allows imaging the wake flowsfrom both the dorsal and anal fins at the same time. as wellas fin and body position relative to the light sheets.Inducing fish to swim in such a restricted position in theflow tank can be difficult and time-consuming, but carefulselection of sequences and accurate fish positioning is vitalto obtaining accurate data on fin hydrodynamic function. While time-resolved two-dimensional PIV with high-speed cameras and continuous lasers has been used to studyfin function in several species of fishes to date (Druckerand Lauder 2000, 2003; Liao and Lauder 2000; Muilleret al. 2000; Nauen and Lauder 2002a; Tytell 2004; Tytelland Lauder 2004; Wilga and Lauder 1999, 2000, 2001),three-dimensional information on fish fin flow patterns ishighly desirable. Such data can be obtained in part by usingstereo PIV (Nauen and Lauder 2002b) or using multiplelight sheet orientations (Drucker and Lauder 1999), buteven this approach only generates the three vector com-ponents of flow confined to one or more narrow (1-2 mmthick) planes. Reconstructions of three-dimensional flowpatterns then requires piecing together data from severaldifferent fin beat cycles which is difficult as freely swim-ming fishes often do not move their fins in precisely thesame manner from stroke to stroke or maintain strict con-trol of body position. Phase averaging of fin PIV data fromfreely swimming fishes is possible (e.g., Tytell and Lauder2004) but often difficult to do without introducing con-siderable variation into the data. In Sect. 6 below we describe data obtained on thehydrodynamics of the bluegill sunfish pectoral fin usingboth scanning PIV, and a transverse-plane PIV approachthat samples flow with high temporal resolution down-stream of swimming fish with cameras that view the wakefrom behind (Fig.3). These approaches, especially whenused in conjunction with each other, provide a reasonablycomplete picture of fin-induced three-dimensional flowpatterns. In scanning PIV, a continuous wave horizontal lightsheet is scanned, using a moving mirror, down through the Fig. 3 Methods for the study of fin hydrodynamics in freelyswimming fishes. a Brook trout swimming between two light sheetsproduced by two continuous wave argon-ion lasers to study thefunction of dorsal and anal fins. High-speed digital video camerasimage the fin wake flow patterns from above and below at the sametime. Image by E. Standen, from Standen and Lauder (2007).b Scanning PIV in which a horizontal laser light sheet is rapidlyscanned vertically through the beating pectoral fin (white arrowsindicate the direction of laser sheet movement) in freely swimmingbluegill sunfish to image fin wake flow patterns. c Experimentalarrangement for stereo PIV using a light sheet transverse toswimming bluegill, downstream of the beating pectoral fin. Top moving pectoral fin and its wake (Fig. 3b). The light sheetscans through 5-10 cm vertical distance in 50-100 ms, andparticle flows are imaged with aa high-speed camera(1,024×1,024 pixel resolution) at 500 Hz from below thelight sheet looking up at the fish and the fin wake. A side-view camera provides data on the position of the light sheetrelative to the fish fin, and gives basic kinematic data on themotion of the fin. Brucker (1997), Rockwell et al. (1993),and Burgmann et al.(2006) provide further discussion ofPIV scanning approaches. In another set ofexperiments, we used a continuous laserlight sheet oriented transversely to the fish body axis andplaced downstream of the fish fin (Fig. 3c). Fin wake flowsmove toward and then through the laser light sheet as the panel shows a schematic top view of the experimental arrangement.Use of three high-speed cameras allows simultaneous imaging ofbody and fin position (camera 1) and stereo PIV of flow through thetransverse light sheet (middle panel, camera #2 and #3). These twocameras were aimed at a mirror downstream of the swimming fish,imaging at 500 Hz, 1/2,000 s shutter speed, and provided data on theu, v, and w components of flow through the transverse laser lightsheet. The bottom panel shows a bluegill swimming in the flow tankwith the transverse light sheet. Bluegill swam with their pectoral fin atvarying distances upstream of the light sheet in different trials (bottompanel image courtesy of E. Tytell) fish maintains position in the flow tank while swimming at aslow pectoral fin swimming speed. With a temporal sam-pling rate of 500-1,000 Hz and short shutter speeds(1/2,000 s or less), stereo PIV images can be obtained ofwake flow patterns moving toward the camera, and recon-structed into a three-dimensional representation of fin flows.Briicker (2001) discusses PIV using a light sheet orientationorthogonal to free-stream flow and issues involved inimaging such flows from downstream. This approach hasthe advantage of imaging the full wake from the bodysurface to a distance of several fin chords away into the free-stream as flow moves into the transverse light sheet. Anadditional feature of these experiments is the use of anothercamera to image fish body position in a side view (Fig. 3c), allowing quantification of fish body acceleration during thefin beat synchronously with the stereo PIV images. Asshown in Fig. 3c, we used red light to illuminate theswimming fish, and a Photron PCI 1024 high-speed digitalvideo camera with a highpass filter on the lens to allow redlight through but block green light from the continuouswave argon-ion laser. This camera (#1 in Fig. 3c) imagedbody position and fin movement. Two additional identicalsynchronized Photron cameras (#2 and #3 in Fig. 3c) wereaimed in stereo configuration with Scheimpflug adapters ata mirror in the flow tank downstream from the swimmingfish. These two cameras were focused onto the laser lightsheet located upstream of the mirror (Fig.3c) and hadlowpass filters on each lens to block red light but allow thegreen light from the argon-ion laser through to the camerasensors. This experimental arrangement allows simultaneous acquisition of body and fin position through time andfin wake flows in stereo view. Since fin wake flows advectthrough the laser plane, a three-dimensional view of the finwake can be formed. However, since the wake is sampled atonly one location, any interactions among vortices down-stream of the laser plane will not be visualized. All cameraviews were calibrated and u, v, and w velocity vectorcomponents calculated using Davis 7.1.1 software fromLaVision Inc., Ypsilanti, MI, USA. Multiple replicateexperiments were conducted on individual bluegill sunfish(Lepomis macrochirus) of mean total body length (L) of18 cm swimming at 0.5 Ls. Swimming bluegill naturallypositioned themselves at slightly different positions in theflow tank during the replicate trials, and thus data wereobtained with the fin at different distances upstream of thetransverse light sheet. In most sequences, the posterior re-gion of the body can be seen in the PIV views as the tailextends toward the PIV cameras (Figs. 3c, 7a, 8). PIV se-quences of the pectoral fin wake were obtained of steadyswimming and also a variety of maneuvers, but in this paperwe focus on the steady swimming data. These experimentalanalyses are conducted in conjunction with computationalfluid dynamic analyses of sunfish pectoral fin function(Lauder et al. 2006; Mittal et al. 2006). 4 Overview of dorsal and anal fin function In this section we present kinematic and hydrodynamicdata from fish dorsal and anal fins to illustrate the extent towhich flows generated by these fins modify the hydrody-namic environment experienced by the tail, and as anexample of the hydrodynamics of median fin function. Perhaps the most surprising result to emerge from anal-yses of fish dorsal and anal fin function to date is the extentto which these fins generate side forces. Analyses of medianfin wake flows in both trout and sunfish (Drucker and Lauder 2001b,2005; Standen and Lauder 2005, 2007) showthat although a reasonable amount of thrust may be pro-duced, the majority of locomotor force produced by medianfins is directed laterally, to each side (Fig. 4). In bluegillsunfish, the soft dorsal fin generates about one-third of thethrust produced by the tail, and approximately twice asmuch lateral force as thrust. Tytell (2006) estimated thattogether, the dorsal and anal fins in bluegill generate nearlyas much total force as the tail fin. But in rainbow trout,Drucker and Lauder (2005) showed that the dorsal fingenerates side forces that are five times thrust force mag-nitudes, and that the dorsal fin produces a distinct vortexwake at swimming speeds less than 2.0 body lengths (L) persecond. Figure 4a illustrates the vortex wake of the dorsalfin of a rainbow trout swimming at 1 Ls. Strong pulses offluid momentum directed to each side are generated as the A Dorsal fin Caudal fin B Adipose fin light sheets C Pectoral fin Anal fin B Vorticity (rad) Fig. 4 Median fin hydrodynamics in swimming trout. a Schematic toshow the position of dorsal and anal fins in trout and the position ofthe laser light sheets in panels B and C. Trout have an additional smallmedian fin, the adipose fin, that is not actively moved. b Dorsal finwake (yellow arrows) in rainbow trout swimming steadily at 1.0 Ls.The dorsal fin is to the left and the tail to the right. Note thealternating lateral jet flows shed by the dorsal fin. The red dots showthe path of the tail, which moves through the centers of vortices shedby the dorsal fin (from Drucker and Lauder 2005). c Anal fin wakefrom a brook trout swimming steadily at 0.5 Ls. Strong lateralmomentum is shed into the wake by the anal fin, and the tail movesthrough this vortex wake (from Standen and Lauder 2007) dorsal fin sweeps back and forth, and the tail passes throughthe centers of the shed vortices. At higher swimming speedsabove 2.0 Ls,however, dorsal fin amplitude decreases andno wake is formed: the dorsal fin is most active during slowswimming and appears to be inactive during more rapidlocomotion (Drucker and Lauder 2005). Standen and Lauder (2007) studied the function of bothdorsal and anal fins in swimming brook trout, and found thatboth fins generate significant wake vorticity that is directedto the same side of the fish resulting in balanced roll torques(Fig. 4b). Neither the dorsal nor the anal fins generate sig-nificant thrust, while both fins produce nearly synchronousside momentum jets even though the dorsal and anal fins arelocated at different longitudinal positions on the trout.Differences in dorsal and anal fin shape and heave and pitchmotions combine to result in temporally coincident jetformation which in turn balances roll torques. The observation that the caudal fin moves through thedorsal and anal fin wake suggests that, if the motion of thecaudal fin is phased appropriately, additional thrust may beobtained resulting from the increase in angle of attack atthe tail that results from the change in free-stream flowgenerated by the dorsal and anal fins (Drucker and Lauder2001b; Standen and Lauder 2007). Computational fluiddynamic analysis of two foils in series using the pattern ofmotion from the sunfish dorsal fin and tail (Akhtar et al.2007), showed that indeed considerable increases in thrustare realized by the sunfish tail as a direct result of thedorsal fin wake initiating the formation of a strongerleading edge vortex on the tail than would otherwise bepresent. Interestingly, the phase difference between thesunfish dorsal fin and tail (108°) was not the optimal phasepossible. Exploration of the phase parameter space showedthat a phase of 48° produced optimal thrust enhancementby the tail, although this is a phase relationship never seenin a swimming sunfish due to the coupling between thedorsal fin and tail through the body. Experimental data on median fin hydrodynamics infishes (Fig. 4) indicate that the these fins play a substantialrole in the maintenance of body stability, in modifying theflow environment encountered by the tail, and, in the caseof sunfish, generating thrust during steady rectilinear pro-pulsion. There is a considerable diversity of median finstructure in fishes, and yet the hydrodynamic significanceof this variation is as yet unknown, and there are a plethoraof questions for future experimental hydrodynamic re-search on the median fins of fishes. 5 Pectoral fin function: kinematics A conspicuous feature of biological propulsion in fluids(especially water) is flexibility of the thrust-generating surfaces, and bending and twisting of control surfaces isespecially evident when fishes swim at slow speeds usingonly their fins (and not body deformation) to produce thrust.In particular, the pectoral fin swimming gaits of bluegillsunfish (Fig.5) illustrate how fish fins deform during pro-pulsion, and the complexity of this deformation. Whenswimming steadily at slow speeds (less than 1 Ls ), bluegillsunfish use the paired pectoral fins almost exclusively. Onthe outstroke, both the upper and lower edges of the fin moveaway from the body wall in a nearly simultaneous motionwhich results in the fin achieving a“cupped”configuration(Fig. 5b, e): the pectoral fin has two leading edges. Fin areaincreases during the outstroke, and the upper third of the finbends into a wave that travels spanwise from fin root to tip ata speed faster than the swimming speed: hence this travelingwave along the upper fin margin appears to contribute tothrust. The maximum“cupped”configuration is achieved atabout 75% of the outstroke as the upper and lower fin edgesmove toward each other. Minimum fin area occurs at the timethe fin transitions from the outstroke to the return stroke. Early in the retraction stroke of the pectoral fin, theupper fin wave has progressed nearly two-thirds of the wayalong the fin span and causes a “dimpling” of the finsurface behind the upper edge (Fig. 5f) which appears tostabilize the vortex formed on the upper fin edge (Lauderet al. 2006). On the return or retraction stroke, fin areaincreases and the fin moves rapidly back to lie flat along thebody. There is often an extended time in between fin beatsduring which the fin is held along the body wall (Gibb et al.1994) before the next beat begins. Pectoral fin kinematics in bluegill sunfish are broadlyrepresentative of how many bony fishes use their pectoralfins using a complex and time-varying combination of lift-and-drag forces to generate thrust. But some species exem-plify more clearly the ends of the lift-and-drag continuum,and use primarily drag-based propulsion (Walker 2004) orlift-based mechanisms (Walker and Westneat 1997). Fin kinematics during maneuvering locomotion aresubstantially different from propulsion, and may involvemuch more substantial bending of fin rays, greater angularexcursions, and dramatic differences between pectoral finconformation on the left and right sides of the body duringa turn (Drucker and Lauder 2001a; Higham et al. 2005;Lauder et al. 2006). This contrasts with patterns of wingmotion in birds and insects where left-right differencesin wing kinematics during turns are relatively slight(Dickinson 2005; Warrick et al. 1998). 6 Pectoral fin function: hydrodynamics A C Fig. 5 Pectoral fin kinematics in bluegill sunfish (18 cm L) swimming at 0.5 Ls-. Panels A-D show four times during a single fin beatcycle. Images are from 250 Hz digital video (1,024×1,024 pixelresolution) taken from behind the swimming bluegill lookingupstream. Yellow arrows show the major movement patterns of thefin, while red and blue arrows point to the upper (dorsal) and lower(ventral) margins of the fin, respectively. a Pause phase when thepectoral fin is held against the body, prior to the start of the fin beatcycle. b Middle of the fin outstroke (“downstroke") showing thecupped configuration of the fin in which both the upper and lower finrays move out from the body together, forming two leading edges. c Twisting of the fin at the transition between outstroke and returnstroke. d Middle of the return stroke (“upstroke”) during which thefin is expanded and pulled back toward the body (axis labels in thispanel apply to panels A-D). e Enlarged view of the fin at the positionshown in panel B to show details of the cupped fin conformation andthe positions of the individual fin rays. f Enlarged view of the fin inside view slightly prior to the image in panel C to show the wave ofbending that passes out the upper third of the fin from root to tip. Notethe “dimple” formed behind the upper edge of the fin (green arrow)and variation in conformation of the different fin rays at this time induced by fin motion can also be complex. PIV has beenused to understand the hydrodynamic effect and forceproduction of pectoral fin movement during both propul-sion and maneuvering in a diversity of fishes (sharks, sturgeon, bluegill sunfish, and trout, Drucker and Lauder1999, 2000, 2001a,2002b,2003; Lauder et al. 2006; Wilgaand Lauder 1999, 2000, 2001), but these studies have todate relied on more traditional PIVapproaches using two-dimensional laser light sheets, usually oriented hori-zontally (perpendicular to the body axis) or vertically(parallel to the fish body). Two useful modifications to thetraditional PIV approach (Fig.3) are (1) to scan a laserlight sheet through the pectoral fin and its wake, and (2) touse a light sheet in a transverse, orthogonal orientation tofree-stream flow and image flow from downstream. Boththe modifications of the usual PIV approach provideincreased three-dimensional information on the hydrody-namic consequences of fin function. Scanning PIV of the bluegill sunfish pectoral fin wake(Fig. 6) demonstrates that during the fin outstroke (when itis moving away from the body), water is accelerated bothdownstream and laterally, to the side. Because the fin istranslucent, flow structures can be resolved in between thefin and the body, and water between the body and fin isaccelerated on the outstroke (also see Lauder et al. 2006).At the end of the outstroke (Fig. 6b) a vortex ring has beenshed with a central momentum jet directed back and to theside. As the pectoral fin returns to the body on the instroke,a vortex loop is shed (Fig. 6c). There is a significantchangeinnddirection(nearlyy90°)tbetweenntthesidemomentum added to the water on the outstroke and thereturn stroke of the fin which is clearly evident in the wakeshown in Fig. 6c. Analysis of pectoral fin hydrodynamics using transverseplane stereo PIV (Fig. 7) shows that on the fin outstroke alarge volume of water is accelerated beyond free-streameven though much of the fin is moving away from the body.Kinematic analysis (Fig. 5b) shows that on the outstrokethe upper third of the fin is oriented down and back, andthis region of the fin could thus generate thrust during theoutstroke (Fig.7). Transverse plane PIV at the level of the pectoral finshows that on the fin downstroke (data not shown here),the kinematic cupping of the fin in which both the upperand lower fin rays move away from the body simulta-neously (Fig. 5b), produces dual leading edge vortices(Lauder et al. 2006). The simultaneous presence ofopposite sign vortices on the pectoral fin may act tominimize vertical body oscillations compared to a heav-ing and pitching foil in which significant momentum isadded to the water orthogonal to the free-stream on eachhalf stroke. On the instroke (Fig. 8), flow moves back toward thebody and is directed initially down and medially, and laterin the instroke almost directly medially. Throughout theinstroke, a large region of flow is accelerated beyond free-stream (Fig.8). Figure 9 shows the result of calculations of momentumflux through the transverse laser plane to give single finforces resolved throughout one fin beat cycle. Vertical andside forces generated by the fin average between 1 and Fig. 6 Scanning PIV results from pectoral fin locomotion in abluegill sunfish (L= 18 cm) swimming at 0.5 Ls(see Fig. 3b formethods). The laser light sheet scanned from top to bottom, and wakeflows were imaged from below at 500 Hz. Images show the wakeflow patterns early in the fin outstroke (a), at the transition fromoutstroke to instroke (b), and after the fin has reached the bodysurface at the end of the instroke (c). Mean free-stream flow has beensubtracted. Note especially the added momentum in the downstreamand side directions on the fin outstroke, and the reversal of momentumseen in panel C between the outstroke and instroke, visible as the twonearly orthogonal regions of jet flow downstream of the pectoral fin.Axes in panel A apply to all panels 5 mN and there is only a small deviation from the initialvalues at the start of the beat. Fish often drift slightlyduring pectoral fin propulsion, and hence side and verticalforces may not return exactly to their initial values. Thrustforce per fin peaks at about 2 mN, and the fin clearlygenerates thrust throughout the beat (Fig. 9c). Some tem-poral smoothing in the force trace may result from the factthat the transverse laser light sheet was ~0.5 L downstreamfrom the pectoral fin. x B Fig. 7 PIV data imaged in the transverse plane (see Fig. 3c formethods) looking upstream at the wake shed by the pectoral fin ofbluegill sunfish during locomotion at 0.5 Ls.This figure shows datafrom the fin near the end of the outstroke. a Water velocity in the xyplane showing the u and v components of flow, with the w component(z-direction) illustrated as a contour plot in the background. Red colorindicates water moving faster than mean free-stream flow (9 cm s)as a result of pectoral fin motion, while blue indicates flow slowed toless than free-stream. The tail extends toward the camera in themiddle right of the image, and vectors near the tail have been masked.Note the large region of red indicating that the pectoral fin outstrokehas accelerated water in the wake to beyond free-stream velocity.b Three-quarter view of the pectoral fin wake flow at the same time asin panel A to show vorticity and velocity greater than free-stream.Green color indicates zero vorticity, blue negative vorticity (maxi-mum 10 s ), and red positive vorticity (maximum 20 s-). Note thetwo counter rotating centers of flow separated by fluid acceleratedbeyond free-stream This pattern of pectoral fin thrust production measuredexperimentally in sunfish is consistent with that calculatedusing CFD based on sunfish pectoral fin kinematics (Mittalet al. 2006): the pectoral fin generates thrust throughout thefin beat. This result contrasts with data from many previous VelocityVz (m/s) 0.14 0.10 0.07 X Fig. 8 PIV data imaged in the transverse plane (see Fig. 3c formethods) looking upstream at the wake shed by the pectoral fin ofbluegill sunfish during locomotion at 0.5 Ls. This figure shows datafrom the fin at mid-instroke (a) and late instroke (b). In both panelswater velocity is shown in the xy plane (u and v components) of flow,with the w component (z-direction) illustrated as a contour plot in thebackground. Red color indicates water moving faster than mean free-stream flow (9cm s) as a result of pectoral fin motion, while blueindicates flow slowed to less than free-stream. The tail extends towardthe camera in the middle right of the images, and vectors near the tailhave been masked. a Note the change in direction of side velocitycompared to the fin outstroke in Fig.7 and the large region ofaccelerated flow. b Late in the instroke the pectoral fin wake isoriented toward the body and a region of accelerated flow is stillevident experiments and computational work on forces generatedby heaving and pitching foils, in which there is a period ofnet drag force produced as foils reverse direction at theextremes of the stroke cycle. For example, data on flapping 5 A 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 Time(s) Fig. 9 Wake forces (for one fin) from bluegill sunfish pectoral finlocomotion at 0.5 Ls-estimated in three dimensions from transverselight sheet stereo PIV data (see Fig. 3c for methods,) calculated byestimating the momentum flux through the transverse light sheetplane, and knowing the weight of the fish. Imaging wake flows at500 Hz in this plane allows estimation of momentum flux throughtime.Values should be doubled to estimate total force on the fish fromboth pectoral fins. There was negligible activity in other fins duringthis sequence, so total locomotor force on the bluegill can beattributed almost exclusively to the pectoral fins. There is little changein the mean side (a) or vertical force (b) throughout the fin beatcompared to the initial values before the fin beat begins. Smalldifferences in force to the left and right or up and down reflect slightchanges in fish position during the fin beat. Note that the pectoral fingenerates thrust throughout the fin beat cycle (c); there is no timewhen the pectoral fin produces net drag (negative Fz). Also see Penget al. (2007), Drucker and Lauder (1999), and Mittal et al. (2006) forother methods of estimating locomotor force from fish pectoral fins foils by Read et al. (2003), computations of foil thrustcoefficients by Dong et al. (2006) and Guglielmini andBlondeaux(2004), all show a time of net drag at strokereversal. A computational fluid dynamic analysis of thepectoral fin of a wrasse, a fish with a more flapping foil-likefin stroke (Ramamurti et al. 2002), also shows a significantperiod of drag at the fin reversal, and experimental esti-mates of thrust coefficients in fishes using flapping androwing propulsion (Walker and Westneat 2002; Walker2004) show long periods of drag. In contrast, the datapresented here and our previous experimental and com-putational analyses of the sunfish pectoral fin whichexhibits a more complex movement pattern than simplerowing or flapping, showsthat thrustisgeneratedthroughout the fin beat and that there is no time in the finbeat cycle when net drag is produced (Lauder and Madden2006; Lauder et al. 2006; Mittal et al. 2006). Our observation of continuous thrust by the sunfishpectoral fin is understandable in terms of fin kinematics, which contrast with those of rowing and flapping fishesstudied previously. Movement of the sunfish pectoral fin,with the cupping shape on the outstroke, the flexible finsurface, the outer third of which is oriented downstream onthe outstroke, the wave of bending that passes along theupper third of the fin, area minimization at the transitionbetween downstroke and upstroke, and area expansion onthe return stroke, all combine to generate continuous thrustthroughout the beat (Fig.5). Many features of this kine-matic pattern are general components of fish fin function(Fish and Lauder 2006; Lauder 2006), and this resultsuggests that one important role for flexibility of the pro-pulsive fin surface in fishes is to modulate force productionand smooth out oscillations that might arise at transitionsduring the movement cycle. Bending of the fin surface andof individual fin rays allows continued thrust production byat least some portion of the fin at all times during the finstroke that is sufficient to overcome drag produced by otherfin regions. 7 Conclusions and prospectus The experimental study of fish locomotion has undergone arenaissance in recent decades as new technologies forvisualizing fin and body movement and for quantifyingwater flow produced by the body and fins have matured andbecome more widely available to biologists. Studyingfreely swimming fish moving under controlled conditionsin laboratory flow tanks has allowed detailed analyses offin deformation, the role of flexibility in generating pro-pulsive forces, and the forces produced by flexible fins.Fish fins are remarkable in having active camber controland the consequent ability to resist hydrodynamic loading.Furthermore, fin-fin hydrodynamic interactions are sub-stantial, especially among median fins. There are currentlyno experimental data that suggest an interaction betweenpaired fins and median fins, although such interactions arecertainly possible and remain to be demonstrated. Experimental analyses of living fishes are, by their verynature, limited to studying what nature provides in the wayof fin position, structure, and activation pattern. Althoughsurgical modifications of fish fin shape are possible (e.g.,Webb 1977), such modifications provide for only a rela-tively limited range of shape changes, and do not permitmajor alterations in fin position or movement pattern, andfish may alter the way fins are moved post-surgically.Another approach, and one that allows for much morecontrol over movement pattern and for greater explorationof the parameter space of fin phase, frequency, andamplitude is to use robotic models of fish fins (Kato 1998,2000;Lauder et al. 2007; Tangorra et al. in press; Trian-tafyllou and Triantafyllou 1995; Triantafyllou et al. 2004). Both robotic models of a specific fin type such as thepectoral fin, and the use of more abstract robotic modelssuch as dual-flapping foils to approximate median fininteractions, provide invaluable flexibility for understand-ing how fish fins function. Analyses of such robotic sys-tems will likely prove to be an important path for futurework on fish fin function. Finally, we anticipate that computational fluid dynamicapproaches will increasingly use as input experimentallymeasured fin kinematics to more accurately estimatelocomotor forces, and allow direct comparisonsVwithexperimental force measurements. Such studies are, todate, few (but see Akhtar et al. 2007; Mittal 2004; Mittalet al. 2006; Ramamurti et al. 2002). But the promise of acloser integration of computational approaches, the use ofrobotic models, and increasingly detailed experimentalanalyses of fish fin function, suggests that the next decadewiillll wWlitness major progress in understanding the functionof fish fins, and improved ability to use them to design bio-inspired propulsors for AUVs. Acknowledgments This work was supported by an ONR-MURIGrant N00014-03-1-0897 on fish pectoral fin function, monitored byDr. Thomas McKenna and initiated by Dr. Promode Bandyopadhyay,and by NSF grant IBN0316675 to G.V.L. We thank Drs. Rajat Mittaland Promode Bandyopadhyay for many helpful discussions on bio-inspired propulsion. Dr. Wolf Hanke designed the laser scanningsystem and we are very grateful for his assistance with those exper-iments. Karsten Hartel and Chris Kenaley kindly provided the grouperphotograph in Fig. 1a, Em Standen took the image in Fig. 3a, andEric Tytell provided the bluegill picture used in Fig. 3c. Tony Juliusand Julie Idlet provided invaluable assistance in the lab. Thanks alsoto two anonymous reviewers who provided comments helpful inclarifying the manuscript. References Akhtar I, Mittal R, Lauder GV,Drucker E (2007) Hydrodynamics of abiologically inspired tandem flapping foil configuration. TheorComput Fluid Dyn21:155-170 Alben S, Madden PGA, Lauder GV (2007) The mechanics of activefin-shape control in ray-finned fishes. J R Soc Interface 4:243-256 Anderson J (1996) Vorticity control for efficient propulsion. Ph.D.Thesis, MIT/WHOI, 96-02 Brucker C (1997) 3D scanning PIV applied to an air flow in a motoredengine using digital high-speed video. Meas Sci Technol8:1480-1492 Briicker C (2001) Spatio-temporal reconstruction of vortex dynamicsin axisymmetric wakes. J Fluid Struct 15:543-554 Burgmann S, Brucker C, Schroder W (2006) Scanning PIVmeasurements of a laminar separation bubble. Exp Fluids41:319-326 ( Carling J C, Williams TL, Bo w tell G ( 1 998) Self-propelled anguilli-form s wimming: simultaneous solution o f t h e two-dimensionalNavier-Stokes e quations a nd Newton’s la w s of mo t ion. J E x p Biol 201:3143-3166 ) ( Dickinson M H (2005) The ini t iation a n d co n trol o f ra p id fl i ghtmaneuvers in fruit flies. Int Comput Biol 45:274-281 ) Dong H, Mittal R, Najjar FM (2006) Wake topology and hydrody-namic performance of low aspect-ratio flapping foils. J FluidMech 566:309-343 Drucker EG, Lauder GV (1999) Locomotor forces on a swimmingfish: three-dimensionalvortexwake dynamicsquantifiedusing digital particle image velocimetry. JJBExp Biol202:2393-2412 Drucker EG, Lauder GV (2000) A hydrodynamic analysis of fishswimming speed: wake structure and locomotor force in slowand fast labriform swimmers. J Exp Biol 203:2379-2393 Drucker EG, Lauder GV (2001a) Wake dynamics and fluid forces ofturning maneuvers in sunfish. J Exp Biol 204:431-442 Drucker EG, Lauder GV (2001b) Locomotor function of the dorsal finin teleost fishes: experimental analysis of wake forces in sunfish.J Exp Biol 204:2943-2958 Drucker EG, Lauder GV (2002a) Experimental hydrodynamics of fishlocomotion: functional insights from wake visualization. IntComput Biol 42:243-257 Drucker EG, Lauder GV (2002b) Wake dynamics and locomotorfunction in fishes: interpreting evolutionary patterns in pectoralfin design. Int Comput Biol 42:997-1008 Drucker EG, Lauder GV (2003) Function of pectoral fins in rainbowtrout: behavioral repertoire and hydrodynamic forces. J Exp Biol206:813-826 Drucker EG, Lauder GV (2005) Locomotor function of the dorsal finin rainbow trout: kinematic patterns and hydrodynamic forces.J Exp Biol 208:4479-4494 Fish F, Lauder GV (2006) Passive and active flow control byswimming fishes and mammals. Ann Rev Fluid Mech 38:193-224 Geerlink PJ, Videler JJ (1974) Joints and muscles of the dorsal fin ofTilapia nilotica L. (Fam. Cichlidae). Neth J Zool 24:279-290 Geerlink PJ(1979) The anatomy of the pectoral fin in Sarotherodonniloticus Trewavas (Cichlidae). Neth J Zool 29:9-32 Geerlink PJ, Videler JJ (1987) The relation between structure andbending properties of teleost fin rays. Neth J Zool 37:59-80 Gibb A, Jayne BC, Lauder GV (1994) Kinematics of pectoral finlocomotion in the bluegill sunfish Lepomis macrochirus. J ExpBiol 189:133-161 Guglielmini L, Blondeaux P (2004) Propulsive efficiency of oscillat-ing foils. Euro J Mech B-Fluids 23:255-278 Higham TE, Malas B, Jayne BC, Lauder GV (2005) Constraints onstarting and stopping:behavior compensates for reduced pectoralfin area during braking of the bluegill sunfish (Lepomismacrochirus). J Exp Biol 208:4735-4746 Jayne BC, Lozada A, Lauder GV (1996) Function of the dorsal fin inbluegill sunfish: motor patterns during four locomotor behaviors.J Morphol 228:307-326 Kato N (1998) Locomotion by mechanical pectoral fins. J Mar SciTechnol 3:113-121 Kato N (2000) Control performance in the horizontal plane of a fishrobot with mechanical pectoral fins. IEEE J Oceanic Eng25:121-129 Kern S, Koumoutsakos P (2006) Simulations of optimized anguilli-form swimming. J Exp Biol 209:4841-4857 Lauder GV (2000) Function of the caudal fin during locomotion infishes: kinematics, flow visualization, and evolutionary patterns.Am Zool 40:101-122 Lauder GV, Drucker EG (2002) Forces, fishes, and fluids: hydrody-namic mechanisms of aquatic locomotion. News Physiol Sci17:235-240 Lauder GV, Drucker EG (2004) Morphology and experimentalhydrodynamics of fish fin control surfaces. IEEE J Oceanic Eng29:556-571 Lauder GV (2006) Locomotion. In: Evans DH, Claiborne JB (eds)The physiology of fishes, 3rd edn. CRC, Boca Raton, pp 3-46 ( Lauder GV, Madden PGA (2006) Learning from fish: kinematics andexperimental hydrodynamics f or r oboticists . In t J Automat Comput 4:325-335 ) Lauder GV, Madden PGA, Mittal R, Dong H, Bozkurttas M (2006)Locomotion with flexible propulsors I: experimental analysis ofpectoral fin swimming in sunfish. Bioinsp Biomimet 1: S25-S34 Lauder GV, Tytell ED (2006) Hydrodynamics of undulatory propul-sion. In: Shadwick RE,Lauder GV (eds) Fish biomechanics vol23 in fish physiology. Academic, San Diego, pp 425-468 Lauder GV, Anderson EJ, Tangorra J, Madden PGA (2007) Fishbiorobotics: kinematics and hydrodynamics of self-propulsion.J Exp Biol 210 (in press) Lauder GV, Madden PGA (2008) Advances in comparative physi-ology from high-speed imaging of animal and fluid motion. AnnRev Physiol 70 (in press) Liao J, Lauder GV (2000) Function of the heterocercal tail in whitesturgeon: flow visualization during steady swimming andvertical maneuvering. J Exp Biol 203:3585-3594 Liao J, Beal DN, Lauder GV, Triantafyllou MS (2003) The Karmangait: novel body kinematics of rainbow trout swimming in avortex street. J Exp Biol 206:1059-1073 Mittal R (2004) Computational modeling in biohydrodynamics:trends, challenges, and recent advances. IEEE J Oceanic Eng29:595-604 Mittal R, Dong H, Bozkurttas M, Lauder GV, Madden PGA (2006)Locomotion with flexible propulsors II: computational modelingand analysis of pectoral fin swimming in sunfish. BioinspBiomimet 1: S35-S41 Muller UK, Stamhuis EJ, Videler JJ (2000) Hydrodynamics ofunsteady fish swimming and the effects of body size: comparingthe flow fields of fish larvae and adults. J Exp Biol 203:193-206 Nauen JC, Lauder GV (2002a) Hydrodynamics of caudal finlocomotion by chub mackerel, Scomber japonicus (Scombridae).J Exp Biol 205:1709-1724 Nauen JC, Lauder GV (2002b) Quantification of the wake of rainbowtrout (Oncorhynchus mykiss) using three-dimensional stereo-scopic digital particle image velocimetry.J Exp Biol 205:3271-3279 Peng J, Dabiri JO, Madden PG, Lauder GV (2007) Non-invasivemeasurement of instantaneous forces during aquatic locomotion:a case study of the bluegill sunfish pectoral fin. J Exp Biol210:685-698 Ramamurti R, Lohner R, Sandberg WC (1999) Computation of the3-D unsteady flow past deforming geometries. Int J ComputFluid Dyn 13:83-99 Ramamurti R, Sandberg WC, Lohner R, Walker JA, Westneat M(2002) Fluid dynamics of flapping aquatic flight in the birdwrasse: three-dimensional unsteady computations with findeformation. J Exp Biol 205:2997-3008 ( Read DA, H o ver FS, Triantafyllou MS (2003) Fo r ces on os c illatingfoils for propulsion and maneuvering. J Fluid Struc t 17:163-183 ) Rockwell D, Magness C, Towfighi J, Akin O, Corcoran T (1993)High image-density particle image velocimetry using laserscanning techniques. Exp Fluids 14:181-192 Shadwick R, Gemballa S (2006) Structure, kinematics, and muscledynamics in undulatory swimming. In: Shadwick RE, LauderGV (eds) Fish biomechanics vol 23 iin fish physiology.Academic, San Diego, pp 241-280 ( Standen EM, L auder GV (2 0 05) Do r sal and anal fi n f u nctionin b luegill sunfish (Lepomis m acrochirus): t hree-dimensional ) kinematics during propulsion and maneuvering. J Exp Biol205:2753-2763 Standen EM, Lauder GV (2007) Hydrodynamic function of dorsal andanal fins in brook trout (Salvelinus fontinalis). J Exp Biol210:325-339 Syme DA (2006) Functional properties of skeletal muscle. In:Shadwick RE, Lauder GV (eds) Fish biomechanics vol 23 in fishphysiology. Academic, San Diego,pp 179-240 Tangorra JL, Davidson SN, Hunter IW, Madden PGA, Lauder GV,Dong H, Bozkurttas M, Mittal R (in press) The development ofa biologically inspired propulsor for unmanned underwatervehicles.IEEE J Oceanic Eng Triantafyllou MS, Triantafyllou GS (1995) An efficient swimmingmachine. Sci Am 272:64-70 Triantafyllou MS, Techet AH, Hover FS (2004) Review of experi-mental work in biomimetic foils. IEEE J Oceanic Eng 29:585-594 Tytell ED (2004) Kinematics and hydrodynamics of linear acceler-ation in eels, Anguilla rostrata. Proc R Soc Lond B 271:2535-2540 Tytell ED, Lauder GV (2004) The hydrodynamics of eel swimming. I.Wake structure. J Exp Biol 207:1825-1841 Tytell ED (2006) Median fin function in bluegill sunfish, Lepomismacrochirus: streamwise vortex structure during steadyswimming. J Exp Biol 209:1516-1534 Walker JA, Westneat MW (1997) Labriform propulsion in fishes:kinematics of flapping aquatic flight in the bird wrasseGomphosus varius (Labridae). J Exp Biol 200:1549-1569 Walker JA, Westneat M (2002) Kinematics, dynamics, and energeticsof rowing and flapping propulsion in fishes. Int Comput Biol42:1032-1043 Walker JA (2004) Dynamics of pectoral fin rowing in a fish with anextreme rowing stroke: the threespine stickleback (Gasterosteusaculeatus). J Exp Biol 207:1925-1939 Warrick DR, Dial KP, Biewener AA (1998) Asymmetrical forceproduction in the maneuvering flight of pigeons. Auk 115:916-928 Webb PW (1977) Effects of median fin amputation on fast-startperformance of rainbow trout (Salmo gairdneri). J Exp Biol68:123-135 Wilga CD, Lauder GV (1999) Locomotion in sturgeon: function ofthe pectoral fins. J Exp Biol 202:2413-2432 ( Wilga CD, L auder GV (2 0 00) Three-dimensional kinematics a n d wake s t ructure of the p e ctoral f i ns during locomotion in l eopardsharks Triakis semifasciata . J Exp Bio l 203:2261-2278 ) Wilga CD, Lauder GV (2001) Functional morphology of the pectoralfins in bamboo sharks, Chiloscyllium plagiosum: benthic versuspelagic station holding. JMorphol 249:195-209 ( Wilga C D, L a uder GV (2002) Function of t he het e rocercal ta i l i n sharks: quantitative wake d ynamics d u ring s t eady ho r izontalswimming and vertical maneuvering. J Exp Biol 205:2365-2374 ) Winterbottom R (1974) A descriptive synonymy of the striatedmuscles of the Teleostei. Proc Acad Natl Sci Philos 125:225-317 ( Wolfgang MJ, Anderson J M , Grosenbaugh M, Yue D, T riantafyllouM (1999) Near-body flow d ynamics in s wimming fish. J Exp Biol 202:2303-2327 ) ( Zhu Q , Wolfgang M J , Yue DK P , Triantafyllou G S (2 0 02) Three-dimensional f l ow st r uctures an d vor t icity control in f ish - likeswimming. J Fluid Mech 468:1-28 ) Springer
关闭-
1/13

-
2/13
还剩11页未读,是否继续阅读?
继续免费阅读全文产品配置单
北京欧兰科技发展有限公司为您提供《鱼的运动中柔性类箔鳍的动力学与流体力学研究检测方案 》,该方案主要用于其他中柔性类箔鳍的动力学与流体力学研究检测,参考标准《暂无》,《鱼的运动中柔性类箔鳍的动力学与流体力学研究检测方案 》用到的仪器有德国LaVision PIV/PLIF粒子成像测速场仪。
我要纠错
推荐专场
相关方案





 咨询
咨询