方案详情文
智能文字提取功能测试中
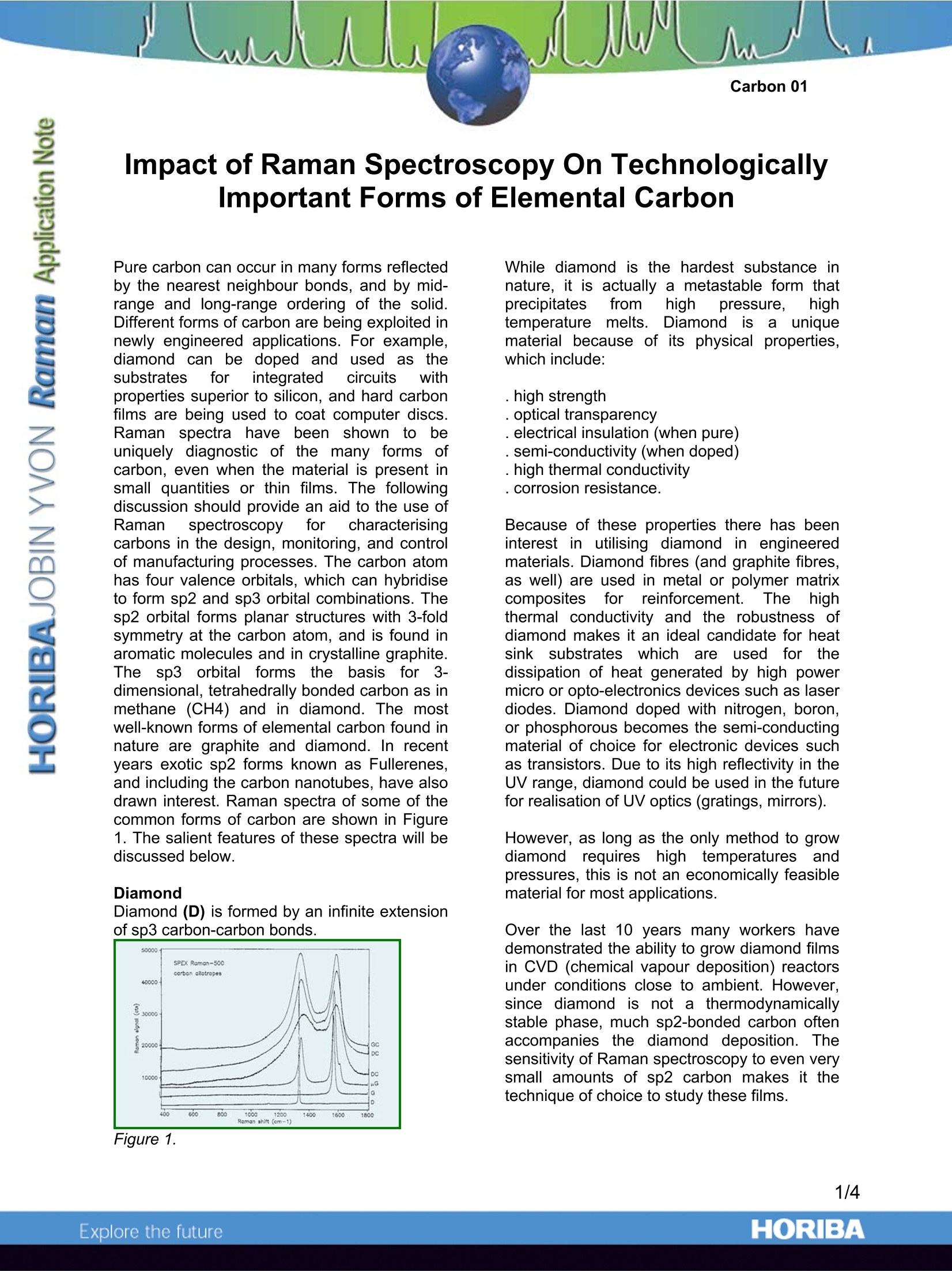
Impact of Raman Spectroscopy On TechnologicallyImportant Forms of Elemental Carbon Pure carbon can occur in many forms reflectedby the nearest neighbour bonds, and by mid-range and long-range ordering of the solid.Different forms of carbon are being exploited innewly engineered applications. For example,diamond can be doped and l iused as thesubstrates for integrated ccircuits withproperties superior to silicon, and hard carbonfilms are being used to coat computer discs.Raman spectra have beenn shown to beuniquely diagnostic of the many forms ofcarbon, even when the material is present insmall quantities or thin films. The followingdiscussion should provide an aid to the use ofRaman spectroscopyforcharacterisinacarbons in the design, monitoring, and controlof manufacturing processes. The carbon atomhas four valence orbitals, which can hybridiseto form sp2 and sp3 orbital combinations. Thesp2 orbital forms planar structures with 3-foldsymmetry at the carbon atom, and is found inaromatic molecules and in crystalline graphite.The sp3 orbital forms; the basis for3-dimensional, tetrahedrally bonded carbon as inmethane (CH4) and in diamond. The mostwell-known forms of elemental carbon found innature are graphite and diamond. In recentyears exotic sp2 forms known as Fullerenes,and including the carbon nanotubes, have alsodrawn interest. Raman spectra of some of thecommon forms of carbon are shown in Figure1. The salient features of these spectra will bediscussed below. Diamond Diamond (D) is formed by an infinite extensionof sp3 carbon-carbon bonds. Figure 1. While diamond is the hardest substance innature, it is actually a metastable form thatprecipitates from high pressure, hightemperature rmelts. Diamondis auniquematerial because of its physical properties,which include: . high strength . optical transparency . electrical insulation (when pure) . semi-conductivity (when doped) . high thermal conductivity . corrosion resistance. Because of these properties there has beeninterest in utilising diamond in engineeredmaterials. Diamond fibres (and graphite fibres,as well) are used in metal or polymer matrixcomposites for reinforcement.Thehighthermal conductivity and the robustness ofdiamond makes it an ideal candidate for heatsink: substrates which are used for thedissipation of heat generated by high powermicro or opto-electronics devices such as laserdiodes. Diamond doped with nitrogen, boron,or phosphorous becomes the semi-conductingmaterial of choice for electronic devices suchas transistors. Due to its high reflectivity in theUV range, diamond could be used in the futurefor realisation of UV optics (gratings,mirrors). However, as long as the only method to growdiamond requires high temperatures andpressures, this is not an economically feasiblematerial for most applications. Over the last 10 years many workers havedemonstrated the ability to grow diamond filmsin CVD (chemical vapour deposition) reactorsunder conditions close to ambient. However,since diamond is not a thermodynamicallystable phase, much sp2-bonded carbon oftenaccompanies the diamond deposition. Thesensitivity of Raman spectroscopy to even verysmall amounts of sp2 carbon makes it thetechnique of choice to study these films. Aside from the ability of Raman spectroscopyto demonstrate the presence of diamond inCVD films, there is also information in theRaman band of the diamond itself. The Ramanline appears at 1332 cm-1 in single crystaldiamond. Under compressive and】tensilestress in the film, the diamond line will shiftrespectively to lower and higher frequency. Inthese films the line can additionally broadendue to heterogeneity of the sample. Workersusing Raman spectroscopy to characterisethese films can develop a quality index thatmeasures the following characteristics: . Diamond frequency . Diamond linewidth .Sp2 carbon background Figure 2 shows a typical Raman spectrum of adiamond film containing substantial amounts ofnon-diamond carbon. The spectrum has beenband-fit to quantify the contributions of thevarious components. It is also useful to notethat YarbroughandRoy (MRS1988Symposium, Diamonld andDiamond-likeMaterial Synthesis, ed. Johnson, et.al, pp.33-38) have noted that under CVD conditions thatare marginal for the deposition of diamond, anew phase is detected. XRD (x-ray diffraction)inthiss articlee ilndicatesnanocrystallinediamond (nD), but the Raman spectrum iscompletely different from anything that hasbeen seen in other carbons. These materialsshow new diffuse bands at ca. 1140 and 1450cm-1. Figure 2. Graphite Graphite (G) is formed from stacks of infinitelyextended planes of fused aromatic rings. TheT electrons in the planes provide for high electrical conductivity and a 0eV bandgap. Vander Waals bonding between the planes is weakwhich is why the planes can lip, and thematerial can be used as a solid lubricant or asan additive to an oily lubricant (for valves,mechanical seals, sliders...) Figure 1 shows the graphite first order phononat about 1580 cm-1 which is fairly sharp,although not as sharp as that of diamond. Thisline has been documented to move with stressand temperature, and because of the potentialfor heating by laser absorption, stress- andtemperature- induced effects can be difficult toseparate. When the long-range order of the graphitelattice is disrupted, a second line appears atabout 1360 cm-1. In a grinding study Tuinstraand Koenig (J. Chem. Phys. 53, 1126, 1970)showed that the intensity of the peak at 1360cm-1 scaled with the size of the crystal, asderived from XRD (x-ray diffraction). Materialwith shorter range order will be denotedmicrocrystalline graphite (pG). A typicalspectrum is shown in Figure 1.The relativepeak intensities of these two lines at 1580 and1360 cm-1, denoted respectively as G and Dlines in the literature (graphite and defect), canvary enormously. In addition, the D band will shift with excitationwavelength, appearing at lower frequencieswith longer wavelength excitation lasers (Wang,Alsmeyer, and McCreery, RamanSpectroscopy of Carbon Materials: StructuralBasis of Observed Spectra, Chemistry ofMaterials 2, 557-563 (1990). Since the relativeintensity is determined by the degree of longrange order of the lattice, it is not surprisingthat the linewidths of these lines also vary.Consequently, it has in recent years, beendeemed more appropriate to calculate the ratioof the integrated intensities of the lines, usingbandfitting algorithms tosseparate thecomponents (LabSpec and DiskSpecsoftwares, Jobin Yvon) TheRaman spectrum1 offmanyi carbonmaterials, including some films and graphitizedfibres. exhibits such extensive broadening andoverlap between the two bands that only band-fitting would permit appropriate use of the data. Carbons; sshowing thissbehaviour will bedenoted disordered carbon (DC). Spectra oftwo samples are shown in Figure 1. In addition, it has been shown (Rouzaud, et.al.,Thin Solid Films, 105, 75-96,1983) that somecarbon films require a second defect band atabout 1500 cm-1 to flatten the residual of theband-fitted Raman spectra. These authorsattribute this band to the presence of interstitialdefects betweenthe graphitic layersbetween structural units in the layers. Icontrast the D band at 1360 cm-1 is attributedto in-plane defects between basic structuralunits. When band-broadening and overlap in carbonfilms is so extensive that separate bands arenot discernible before the band-fit, the materialis called "diamond-like carbon" (DLC). Thismaterial was originally termed "diamond-like"because of its relative transparency andelectrical insulation, properties that result fromthe limited size range of the graphitic structuralunits and their systems. DLC films can beused as passivation layers in magnetic storagedevices. A material called "Glassy Carbon"(GC) hasthe intensity ratio of the D and G bandsreversed (D band higher), with the bands fairlysharp. Ironically this material is composed ofhighly crystalline regions, but with very smalldimensions (privatecommunication.VWm.White, Penn State U.). A spectrum of glassycarbon is shown in Figure 1.l. It is worthwhile to make another comment onthe dependence of these spectra on excitationwavelength. Because the D and G bands arerelated to different sizes of graphitic structuralunits, enhancement of the two Raman bandsthat depends on the proximity of the laserwavelength to electronic transitions, isexpected to behave differently as the excitationwavelength is changed. Yoshikawa,et.al. (Appl.Phys. Lett.,52,-,1639-1641,1988)) showiAspectra of films with 0 and 30% hydrogenacquired with laser wavelengths between 647and 458 nm. For both types of films, the Dband is enhanced relative to the G band whenexcited with a red laser. At any one wavelength,the D band is more intense in the spectrum ofthe pure carbon film than that of a film with30% hydrogen. 1Engineered Carbons Raman spectra can be used to quantify thedegree of structural order as well as orientationin technologically important carbons. Spectraof carbon fibres manufactured from pitch andPAN(polyacrylonitrile) showed not only adifference in long range order but polarisation-dependent intensities related to the orientationof the graphitic planes in the fibers (Adar andNoether, in Microbeam Analysis-1 983, 269-273). Hard carbon films are being used universallyinthe manufacture of hard discs in11thecomputer industry and on read-write heads.Engineering the parameters of the depositionequipment is controlling the physical andtribological pprrooperties of the films. Ramanspectra are being used in this industry as arapid confirmation of the properties of the filmbecause the spectra have been correlated withthe tribological properties of interest (J. Ager,IEEE Trans. Magn, 1992). Such hard films can also be used to improvethe wear properties of cutting tools andsurgical/scalpel blades, as well as to passivatemedical iimplants. Indeed,their chemicalinertness makes them bio-compatible. Figures 3a and b exhibit spectra of carbonfilms that require a fit with 2 and 3 bandsrespectively.The filmwhose spectrumisshown in Figure 3b hasssomeeinterstitialdefects as discussed above. Buckeyballs or Fullerenes, Nanotubes After the theoretical proposal that 60- and 70-atom clusters of carbon might exist in nature,fullerenes; were isolated from carbon soot(Kroto; et al., Nature 318,162,1985). Actually,it is possible to construct balls, tubes andcapsules of graphitic-like fused aromatic rings.The Raman spectra of some of these materialshave been recordedidffrom films oftipurematerials (Bethune, et al., Chem.Phys.Lett,174, 219, 1990). In contrast to the Ramanspectra of the other forms of carbon, whichcontain at most 3 bands in the fingerprintregion of the spectrum (100-1800 cm-1), someof which can be broad,the Raman spectra ofthese materials contains many bands that arequite sharp. The spectra of carbon nanotubes reflect thestructure of the tubes. That is, the diameterand chiral angle (which is related to how agraphite plane would be rolled into the tubularstructure). An excellent review of Ramanscattering in all types of ca.rbons includes adetailed discussion of nanotubes (Dresselhaus,Pimenta, and Eklund, Raman Scattering inCarbon Materials, Chapter 9 in AnalyticalApplications of Raman Spectroscopy, ed. M.Pelletier, (Blackwell, Oxford, 1999). Because the structure of the carbon nanotubesis recognized to determine the semiconducting/metallic properties of these materials, they have been recognised as potential candidatesto be used in nanoelectronics and in manyfunctional devices such as field emitters. Conclusion The Raman spectra of the various forms ofelemental carbon are very sensitive to the typeof nearestneighbourbonding,and tointermediate and long range order. In manycases Raman spectroscopy is the technique ofchoice for characterisation of carbon materials.Correlation of Raman spectral features withtribologicalproperties can facilitate thedeposition of carbon films. ORIBAExplore the future The Raman spectra of the various forms of elemental carbon are very sensitive to the type of nearest neighbour bonding, and to intermediate and long range order. In many cases Raman spectroscopy is the technique of choice for characterisation of carbon materials. Correlation of Raman spectral features with tribological properties can facilitate the deposition of carbon films.
关闭-
1/4
-
2/4

还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
HORIBA(中国)为您提供《工程碳,硬碳膜,富勒烯,碳纳米管样品中结构,缺陷检测方案(激光拉曼光谱)》,该方案主要用于其他中结构,缺陷检测,参考标准《暂无》,《工程碳,硬碳膜,富勒烯,碳纳米管样品中结构,缺陷检测方案(激光拉曼光谱)》用到的仪器有null。
我要纠错
相关方案


 咨询
咨询