方案详情文
智能文字提取功能测试中
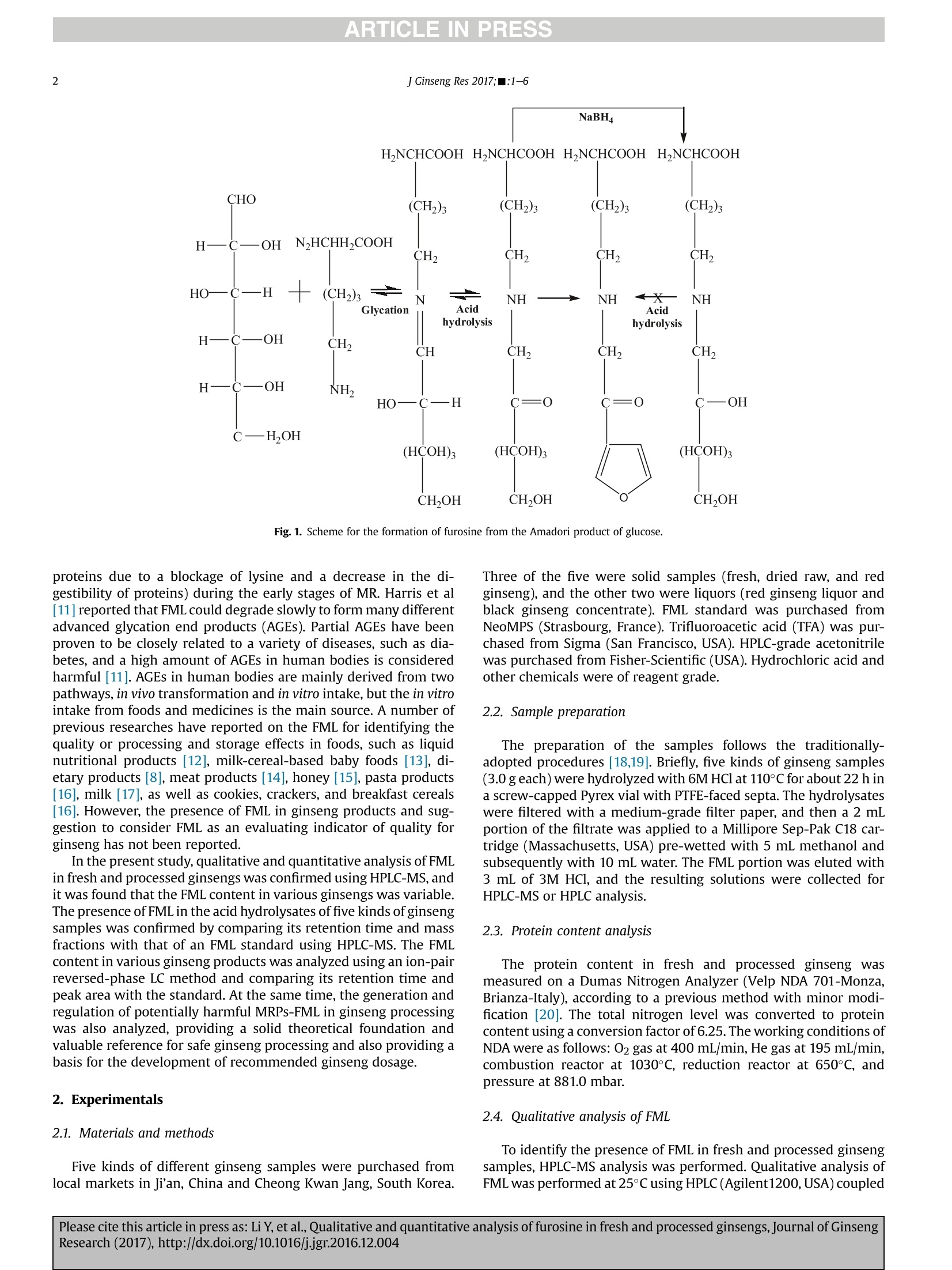
ARTICLE IN PRESS ARTICLE IN PRESS2 Please cite this article in press as: Li Y, et al., Qualitative and quantitative analysis of furosine in fresh and processed ginsengs,Journal of GinsengResearch (2017), http://dx.doi.org/10.1016/j.jgr.2016.12.004 KSG Journal of Ginseng Research journal homepage: http://www.ginsengres.org Research article Qualitative and quantitative analysis of furosine in fresh andprocessed ginsengs Yali Li, Xiaoxu Liu2, Lulu Meng**, Yingping Wang'1,* Institute of Special Wild Economic Animals and Plants, Chinese Academy of Agriculture Sciences, Changchun, China Flight Training Base, Air Force Aviation University, Changchun, China Jilin Province Science and Technology Department, Changchun, China ARTICLEINF O ABSTRACT Article history:Received 5 April 2016Received in Revised form11 November 2016Accepted 7 December 2016Available online xxx Background: Furosine(e-N-2-furoylmethyl-L-lysine, FML) is an amino acid derivative, which is consid-ered to be an important indicator of the extent of damage (deteriorating the quality of amino acid andproteins due to a blockage of lysine and a decrease in the digestibility of proteins) during the early stagesof the Maillard reaction. In addition, FML has been proven to be harmful because it is closely related to avariety of diseases such as diabetes. The qualitative analysis of FML in fresh and processed ginsengs wasconfirmed using HPLC-MS. Keywords:furosineginseng processingquantitative analysishigh-performance liquid chromatography-mass spectrometry Methods: An ion-pair reversed-phase LC method was used for the quantitative analysis of FML in variousginseng samples. Results: The contents of FML in the ginseng samples were 3.35-42.28 g/kg protein. The lowest value wasobserved in the freshly collected ginseng samples, and the highest value was found in the black ginsengconcentrate.Heat treatment and honey addition significantly increased the FML content from 3.35 g/kgprotein to 42.28 g/kg protein. Conclusion: These results indicate that FML is a promising indicator to estimate the heat treatmentdegree and honey addition level during the manufacture of ginseng products. The FML content is also animportant parameter to identity the quality of ginseng products. In addition, the generation and regu-lation of potentially harmful Maillard reaction products-FML in ginseng processing was also investigated,providing a solid theoretical foundation and valuable reference for safe ginseng processing. Copyright @ 2017,The Korean Society of Ginseng, Published by Elsevier. This is an open access article 1. Introduction Ginseng has been consumed as a dietary supplement and herbalmedicine for thousands of years in China, Korea, and Westerncountries [1,2].The processing of ginseng is known to have an in-fluence on its bioactive components and pharmacological activ-ities; therefore, its processing is crucial for ginseng's dietary andmedical functions[33,4]. During the storage (time, humidity, andtemperature) and processing (steaming, drying, and excipientsaddition) of ginseng, reactions between the amino and carbonylgroups often develop randomly. These reactions are called as theMaillard reactions (MRs), amino-carbonyl reactions, or nonenzy-matic model glycation reactions [5,6]. Because abundant carbonyland amino compounds (reducing sugars or ginsenosides withamino acids or proteins) are contained in ginseng, various MRs may occur [7]. MRs in ginseng processing not only produce a largenumber of functional components but also generate a smallamount of harmful substances which cannot be ignored [8]. In2012, planted ginseng was advocated to be“homology of medicineand food” in China within 5 yr, stimulating higher standards withrespect to the quality and safety of ginsengs [9]. ( * Corresponding a u thor. I n stitute of Special Wild Economic Animals an d Plants, Chinese Ac a demy of Agriculture Sciences, Changchun, Jili n 132 1 22, Chi n a. ** Corresponding author. ) J Ginseng Res 2017;■:1-6 NaBH Fig. 1. Scheme for the formation of furosine from the Amadori product of glucose. proteins due to a blockage of lysine and a decrease in the di-gestibility of proteins) during the early stages of MR. Harris et al[11] reported that FML could degrade slowly to form many differentadvanced glycation end products (AGEs). Partial AGEs have beenproven to be closely related to a variety of diseases, such as dia-betes, and a high amount of AGEs in human bodies is consideredharmful [11]. AGEs in human bodies are mainly derived from twopathways, in vivo transformation and in vitro intake, but the in vitrointake from foods and medicines is the main source. A number ofprevious researches have reported on the FML for identifying thequality or processing and storage effects in foods, such as liquidnutritional products [12], milk-cereal-based baby foods [13], di-etary products [8], meat products [14], honey [15], pasta products[16], milk [17], as well as cookies, crackers, and breakfast cereals[16]. However, the presence of FML in ginseng products and sug-gestion to consider FML as an evaluating indicator of quality forginseng has not been reported. In the present study, qualitative and quantitative analysis of FMLin fresh and processed ginsengs was confirmed using HPLC-MS, andit was found that the FML content in various ginsengs was variable.The presence of FML in the acid hydrolysates of five kinds of ginsengsamples was confirmed by comparing its retention time and massfractions with that of an FML standard using HPLC-MS. The FMLcontent in various ginseng products was analyzed using an ion-pairreversed-phase LC method and comparing its retention time andpeak area with the standard. At the same time, the generation andregulation of potentially harmful MRPs-FML in ginseng processingwas also analyzed, providing a solid theoretical foundation andvaluable reference for safe ginseng processing and also providing abasis for the development of recommended ginseng dosage. 2. Experimentals 2.1. Materials and methods Five kinds of different ginseng samples were purchased fromlocal markets in Ji'an, China and Cheong Kwan Jang, South Korea. Three of the five were solid samples (fresh, dried raw, and redginseng), and the other two were liquors (red ginseng liquor andblack ginseng concentrate). FML standard was purchased fromNeoMPS (Strasbourg, France). Trifluoroacetic acid (TFA) was pur-chased from Sigma (San Francisco, USA). HPLC-grade acetonitrilewas purchased from Fisher-Scientific (USA). Hydrochloric acid andother chemicals were of reagent grade. 2.2. Sample preparation The preparation of the samples follows the traditionally-adopted procedures [18,19]. Briefly, five kinds of ginseng samples(3.0 geach) were hydrolyzed with 6M HCl at 110°C for about 22 h ina screw-capped Pyrex vial with PTFE-faced septa. The hydrolysateswere filtered with a medium-grade filter paper, and then a 2 mLportion of the filtrate was applied to a Millipore Sep-Pak C18 car-tridge (Massachusetts, USA) pre-wetted with 5 mL methanol andsubsequently with 10 mL water. The FML portion was eluted with3 mL of 3M HCl, and the resulting solutions were collected forHPLC-MS or HPLC analysis. 2.3. Protein content analysis The protein content in fresh and processed ginseng wasmeasured on a Dumas Nitrogen Analyzer (Velp NDA 701-Monza,Brianza-Italy), according to a previous method with minor modi-fication [20]. The total nitrogen level was converted to proteincontent using a conversion factor of 6.25. The working conditions ofNDA were as follows: O2 gas at 400 mL/min, He gas at 195 mL/min,combustion reactor at 1030°C, reduction reactor at 650°C, andpressure at 881.0 mbar. 2.4. Qualitative analysis of FML To identify the presence of FML in fresh and processed ginsengsamples, HPLC-MS analysis was performed. Qualitative analysis ofFML was performed at 25°C using HPLC(Agilent1200,USA) coupled with 6310 electric spray-ion trap mass spectrometer (Agilent, USA),which consisted of a column oven (G1316A), a pump (G1311A), adegasser (G1322A), and an automatic sampler (G1329A). Thechromatographic separation was performed on a YMC hydrosphereC18 column (4.6×250 mm, 5 um, Tokyo, Japan) and the liquidchromatograph working in electrospray ionization mode underatmospheric pressure and positive polarity (API-ES positive). OtherHPLC-MS conditions were as follows;[7], water/formic acid(99.6:0.4, v/v) at a flow rate of 0.5 mL/min. The ion mode set atselective monitoring was m/z 255, corresponding to FML"[M+H]+". 2.5. Quantitative analysis of FML HPLC analysis of FML was performed at 25°C on an HPLC in-strument (Agilent1200, USA) with a UV detector (G1315D). Theother accessories were as described in section 2.4. An ion-pairreversed-phase (by adding TFA into the mobile phase)- LCmethod was developed for the determination of FML in differentginseng samples. The sample acid hydrolysates were subjected toquantitative analysis, which was performed by the external stan-dard method using a commercial standard of pure FML. Resultswere expressed as g/kg protein, and all the analyses were per-formed in triplicate. The separation of FML was accomplished on a YMC hydrosphereC18 column (4.6×250 mm,5 um, Hewlett-Packard) for the HPLC-MS analysis. Mobile phase consisting of 0.1% TFA in water (A) and0.1% TFA in acetonitrile(B) was applied with the optimized gradientelution as follows: 1-21% B at 0-25 min, and 21-1% B at 25-30 min. Finally, it returned to the initial conditions, setting a bal-ance time of about 10 min before each test for equilibrium of thesystem, which ensures good reproducibility of the method. Theflow rate was maintained at 1 mL/min, and the detection wave-length was set at 280 nm. 3. Results and discussion 3.1. Quantitative analysis of FML 3.1.1. Identification of FML by HPLC-MS The comparison of the retention times and mass fragmentationpatterns for the acid hydrolysates of the ginseng samples withthose of the reference FML established the presence of FML in theginseng samples. Fig. 2 shows the HPLC-MS chromatographs of thereference FML and other samples (standard, fresh ginseng, driedraw ginseng, red ginseng, red ginseng liquor, and black ginsengconcentrate; Figs. 2A-2F). It reached a good resolution for the Fig. 2. Selective ion monitoring of furosine by HPLC-MS in a solution of furosine. (A) Furosine standard. (B) Fresh ginseng. (C) Dried raw ginseng. (D) Red ginseng. (E) Red ginsengliquor. (F) Black ginseng concentrate. Fig. 3. (A) Selective ions monitoring of furosine standard by HPLC-MS. (B) Mass spectrum of the observed furosine fragmentation pattern of furosine standard.(C) Mass spectrum ofthe observed furosine fragmentation pattern of red ginseng. (D) The assignment (or interpretation) of the fragmentation. ( Please cite this article in press as: Li Y, et al.,Qualitative and quantitative analysis of furosine in fresh and processed ginsengs, Journal of Ginseng R esearch (2017), http://dx.doi.org/10.1016/j.jgr.2016.12.004 ) were in good agreement with those of the previous report in foodproducts (pasta, milk, and tigelle bread) [21]. 3.1.2. Confirmation of FML by UV scanning From the retention time of FML at about 10.07 min in ultravioletspectrum, the red ginseng had the same UV absorption spectrumwith the FML standard (Figs. 4A and 4B), and the other four ginsengsamples had the same absorption, further confirming that thechromatographic peak at about 10.07 min corresponds to FML. Theresults further support the presence of FML in the ginseng samples. 3.2. Quantitative analysis of FML 3.2.1. HPLC analysis ofFML A series of FML standard solutions (0.1-1 mmol/mL) werefiltered through a nylon purification kit with a pore size of 0.45-umcut-off (Massachusetts, USA) and then analyzed by the HPLC-pulsed amperometric detection system. TFA was added in themobile phase, which could delay the retention time of highly polarFML on the reversed-phase column. A good linearity was obtainedat concentration of FML in the range of 0.3-10 mg/L with anequation of y=325230x- 12444.4 (R=0.999). The detectionlimit of FML for the method (three signal-to-noise ratio, S/N=3)was 0.05 mg/mL, and the quantitation limit was 0.18 mg/mL. 3.2.2. Quantitative analysis of FML in ginseng samples Fig. 4. UV spectrum of the compound corresponding to the peak at 10.07 min on HPLCfingerprint. (A) Standard. (B) Red ginseng. separation of FML under the HPLC condition. The selective ionmonitoring fraction of mass spectral analysis for the FML standard(Fig. 3A) and the ginseng samples showed the same fragmentationpatterns of 237, 192, 130, and 84 (Figs. 3B and 3C), and the assign-ment of the fragmentation pattern is shown in Fig. 3D, demon-strating the presence of FML in the ginseng samples. These results HPLC results indicated that FML was successfully separated indifferent ginseng samples. Quantitative analyses of FML in freshand differently processed ginseng products (dried raw ginseng, redginseng, red ginseng liquor, and black ginseng concentrate) underthe given HPLC conditions were performed as shown in Fig. 5, andthe data are shown in Table 1. FML was detected in all ginsengsamples at concentrations ranging from 3.35 g/kg protein to42.28 g/kg protein. These values of FML content indicated thatginseng protein was glycosylated to a considerable extent Fig. 5. HPLC fingerprint for furosine analysis in ginseng samples. (A) Standard. (B) Fresh ginseng. (C) Dried raw ginseng. (D) Red ginseng. (E) Red ginseng liquor. (F) Black ginsengconcentrate. Please cite this article in press as: Li Y, et al., Qualitative and quantitative analysis of furosine in fresh and processed ginsengs,Journal of GinsengResearch (2017), http://dx.doi.org/10.1016/j.jgr.2016.12.004 Table 1Contents of FML in different ginseng samples (X±SD) Samples FML content (g/kg protein) Fresh ginseng 3.35±0.18 Dried raw ginseng 10.81 ±0.21 Red ginseng 29.16±1.85 Red ginseng liquor 34.91±0.96 Black ginseng concentrate 42.28±2.78 FML,e-N-2-furoylmethyl-L-lysine compared with other processed foods, such as ultra-high temper-ature milk [13] (310-603 g/kg protein) or processed cheese221(3.5-366.6 g/kg protein). The FML content in fresh ginseng waslow, but it was relatively high in black ginseng concentrate(Table 2). The FML content in processed ginseng products can bevariable depending on many factors, such as processing methods,heating degree, and the excipients; however, fresh ginseng is notexposed to any of these factors. FML is one of the series of MRPsfrom carbonyl and amino compounds, and the processing condi-tions and the auxiliary materials supplemented to the blackginseng concentrate are more feasible to MR, thus a higher level ofFML was obtained. The highest content of FML in black ginsengconcentrate showed that even though honey addition to blackginseng concentrate during the production improved its organo-leptic properties (flavor and taste), the presence of honey alsofavored the MR, and therefore decreased nutritional and medicalvalues, basically through lysine losses. In addition, the content ofFML in freshly-dried raw ginseng, 1-yr-stored dried raw ginseng,and 1.5-yr-stored-dried raw ginseng was 10.81 g/kg protein,12.66 g/kg protein, and 14.78 g/kg protein, respectively, which witha relatively small change. Monitoring the content of FML during theprocessing of red ginseng indicates that the FML generation is slowduring the steaming treatment, thereby giving values of 9.63 g/kgprotein, 13.35 g/kg protein, and 15.69 g/kg protein after 60 min,90 min, and 120 min,respectively, at 95°C. However, according tothe above results, the main process for the formation of FML was airheating (drying). The FML amount increased markedly during theair heating process, reaching values of 24.16 g/kg protein, 28.16 g/kgprotein, and 30.78 g/kg protein after 12 h, 24 h, and 36 h, respec-tively, at 70°C. 3.2.3. Precisions and recoveries The FML concentrations of fresh ginseng, raw ginseng, redginseng, red ginseng liquor, and black ginseng concentrate were Table 2Accuracy of the analytical procedure for FML in red ginseng Samples Original Three Spiked Found Standard Red ginseng (mg/mL) Replicates (mg/mL) (mg/mL) deviation R (%) 0.21 A1 0.20 0.43 0.58 A2 0.20 0.42 A3 0.20 0.42 B1 0.25 0.56 4.73 B2 0.25 0.49 B3 0.25 0.47 C1 0.30 0.53 1.53 C2 0.30 0.54 C3 0.30 0.52 D1 0.40 0.59 2.08 D2 0.40 0.58 D3 0.40 0.62 E1 0.50 0.77 1.53 E2 0.50 0.76 E3 0.50 0.73 FML,e-N-2-furoylmethyl-L-lysine obtained with the precision of the peak area calculation method,and relative standard deviation values of the FML content were1.82%,3.15%, 2.86%, 2.51%, and 2.66% by six parallel measurements,showing good precision. To evaluate the accuracy of the method, the recovery of FMLwasstudied by spiking a mixture of standard FML (1-2.5 times of thesample's concentrate) into the red ginseng sample. According to theresults shown in Table 2, the standard deviations for three repli-cates of each spiked sample of FML in red ginseng were less than 5%and most of them were less than 3%, thus confirming the accuracyof the detection and the absence of matrix effects. 4. Conclusion The present study investigated the qualitative and quantitativeanalysis of FML in fresh and processed ginseng, and the resultsshowed that the lowest FML value was observed in the freshlycollected ginseng sample, and the highest FML value was found inthe black ginseng concentrate. In addition, it was found that theheat treatment and honey addition would increase the FML con-tent. These results show that the content of FML can be a prom-ising indicator to estimate the heat and honey addition levelduring ginseng processing. Lower temperature and lower levelhoney addition should be employed to avoid the generation ofAGEs during the heating process of ginseng and other highpolysaccharides-containing herbal medicines. This research pro-vides useful information for the generation and regulation ofpotentially harmful MRPs in ginseng processing and also offers asolid theoretical foundation and valuable reference for safeginseng processing. In addition, the presence of different values ofFML in various ginseng products for this study can serve as areference for the standard doses of the ginseng available doses perday. Conflicts of interest The authors declare no conflict of interest. Acknowledgments This work was supported by the Project supported by the Na-tional Science Foundation for Youths of China (No.3140101925), theJilin Province Science Foundation for Youths and medicine (No.20150520131JH and 20140311033YY) and the Project of ChangchunKey Technologies Research & Development Program (No.14KG059). References [1] WVan JY, Fan Y, Yu QT, Ge YZ, Yan CP,Alolga RN, Li P, Ma ZH, Qi LW. Integratedevaluation of malonyl ginsenosides, amino acids and polysaccharides in freshand processed ginseng. J Pharm Biomed Anal 2015;107:89-97. [2]Paik DJ, Lee CH. Review of cases of patient risk associated with ginseng abuseand misuse.J Ginseng Res 2015;39:89-93. [3]Jin Y, Kim YJ, Jeon JN, Wang C, Min JW, Noh HY, Yang DC. Effect of White, Redand Black Ginseng on Physicochemical Properties and Ginsenosides. PlantFoods Hum Nutr 2015;70:141-5. 4Lee MH, Lee YC, Kim SS, Hong HD, Kim KT. Quality and antioxidant activityof ginseng seed processed by fermentation strains. J Ginseng Res 2015;39:178-82. ( [5] Davidek T , Clety N, Devaud S , Rober t F, Blank . Simultaneous quantitative analysis of m ai llar d reactio n precursors and pr od ucts b y high-performanceanion exchange ch r omatograp h y.J A gric F ood C h e m 2 0 03;51: 7 259-65. ) [6]Somoza V. Five years of research on health risks and benefits of Maillard re-action products: an update. Mol Nutr Food Res 2005;49:663-72. [7]Du QQ, Liu SY, Xu RF, Li M, Song FR, Liu ZQ. Studies on structures and activitiesof initial Maillard reaction products by electrospray ionisation mass spec-trometry combined with liquid chromatography in processing of red ginseng.Food Chem2012;135:832-8. [8]Sell DR. Ageing promotes the increase of early glycation Amadori product asassessed by s-N-(2-furoylmethyl)-L-lysine (furosine) levels in rodent skin ( collagen T he r e la t ion s hip t o dietary r e striction a nd g lycoxida t i o n. M ech Ageing Dev 1 997;95:81 . ) ( ] Chu n g HS, L ee Y C, K yung Rhee Y, Le e S Y . Consumer acc e ptanc e of gin s engfood prod u cts. J Food Sc i 2011;76:S516 - 22 . ) ( [10] Gokmen V, Serpe n A. Ac a r OC, Morales FJ. Si g nificance of furosine a s h e a t- induced marker i n cookies. J Cereal Sci 2008;48:843- 7 . ) ( [11] H arris CS, Beaulieu LP, Fraser MH , M cIn t yre K L, Owen P L , M a rtin e au L C , C uerr i er A . Johns T , Ha d dad P S , B ennett S A . I n h ibition o f a dvanced g l ycation e a1 nd p roduct f ormation by m e di c inal plant extr a cts c orrelates with p h e n olic m etabolites a nd antioxida n t a c t ivity. Age 2011; 7 7:196. ) [12]McEwen JW, McKenna RJ, O'Kane KA, Phillips RR, Johns PW. Effect of carbo-hydrate DE on blocked lysine and furosine in a liquid nutritional product.Food Chem 2010;119:323-7. ( [13] B osch L, A legr i a A,FarreR, Clemente G. Ef f ect o f storage condit i ons on fu r osine format i on in m i lk - ce r e a l based ba b y f o ods. Food C h em 2008;107:1681-6 . ) ( [14] K eiko Yamaguchi YN.Determination of f u rosine and fluorescence as m a rkers of the maillard reaction fo r t he evaluati o n of meat products during actua l cooking con d itions. Food S ci Technol R es 2 012;18:6 7 . ) [15]Villamiel M, del Castillo MD, Corzo N, Olano A. Presence of furosine in honeys.JSci Food Agri 2001;81:790-3. [16]Garcia Banos JL, Corzo N, Sanz ML, Olano A. Maltulose and furosine as in-dicators of quality of pasta products. Food Chem 2004;88:35-8. [17]Van Renterghem R, De Block J. Furosine in consumption milk and milkpowders. Int DairyJ 1996;6:371-82. [18]Pahm AA, Pedersen C, Stein HH. Application of the reactive lysine procedureto estimate lysine digestibility in distillers dried grains with solubles fed togrowing pigs.J Agric Food Chem 2008;56:9441-6. [19]Boucher S. Pedersen C. Stein H. Schwab C. Evaluation of the furosine andhomoarginine methods for determining reactive lysine in rumen-undegradedprotein. J Dairy Sci 2009;92:3951-8. [20]Juhaimi FA, Ghafoor K, Ozcan MM. Physical and chemical properties,antioxidant activity, total phenol and mineral profile of seeds of sevendifferent date fruit (Phoenix dactylifera L.) varieties. Int J Food Sci Nutr2012;63:84-9. [21]Bignardi C, Cavazza A, Corradini C. Determination of furosine in food productsby capillary zone electrophoresis-tandem mass spectrometry. Electrophoresis2012:33:2382-9. [22]VillamielM, Arias M, Corzo N, Olano A. Survey of the furosine content incheeses marketed in Spain. JFood Protect 2000;63:974-5. 对天然的高丽参和加工后的高丽参中的糠氨酸的定量和定性分析。糠氨酸对人体有毒害作用,和多种疾病的诱因有关,比如糖尿病,所以在对高丽参的加工过程中研究糠氨酸的影响因素非常重要,糠氨酸的含量在新鲜的高丽参中较低,在加工后的高丽参中含量会明显升高。研究表明在加工过程中加热温度和添加蜂蜜的量会影响糠氨酸的含量造成影响。糠氨酸的含量水平可以用于质控或优化高丽参的加工工艺。其中,文中对高丽参的总蛋白含量测定是使用VELP 杜马斯NDA 701完成的。
关闭-
1/6

-
2/6
还剩4页未读,是否继续阅读?
继续免费阅读全文产品配置单
意大利VELP公司为您提供《高丽参中蛋白质检测方案(定氮仪)》,该方案主要用于中药材和饮片中含量测定检测,参考标准《暂无》,《高丽参中蛋白质检测方案(定氮仪)》用到的仪器有VELP唯意朴仪器 杜马斯定氮仪 NDA 702。
我要纠错
推荐专场
定氮仪、凯氏定氮仪、Dumas定氮仪
更多相关方案






 咨询
咨询

