方案详情文
智能文字提取功能测试中
EMERGING AREAwww.rsc.org/obcOrganic & Biomolecular Chemistry Microwave energy: a versatile tool for the biosciences Jonathan M. Collins" and Nicholas E. Leadbeater*b Received 23rd November 2006,Accepted 5th February 2007 First published as an Advance Article on the web 26th February 2007 DOI: 10.1039/b617084f As the range of techniques for microwave heating has expanded, so have the areas in which it can have aprofound impact. Two emerging areas are the application of microwave heating for the synthesis ofpeptides, peptoids, oligopeptides and carbohydrates and in the field of proteomics. Microwave heating is a valuable tool for synthetic chemists. Itis capable of improving product yields and enhancing the rateof reactions as well as being a safe and convenient method forheating reaction mixtures to elevated temperatures.l.2 The fieldhas developed significantly since the first reports of microwave-promoted synthesis in 1986.34 Domestic microwave ovens areincreasingly being replaced by scientific microwave apparatus foruse in synthesis. As well as being safer, these new instruments allowfor accurate control of key parameters such as initial microwavepower, reaction temperature and, in the case of sealed vesselreactions, internal pressure. Microwave heating occurs on a molecular level as opposed torelying on convection currents and thermal conductivity whenusing conventional heating methods. This offers an explanation asto why microwave reactions are so much faster. With microwave ( " CEM Corporation, 3100 Smith Farm Road, M atthews, N C 28104, U SA. E-mail: jonathan.collins @ cem.com; Fax: +1 704 821 7894;Tel: +1 704 821 7 01 5 ) ( D epartment of Che m istry, Un i versity o f Connecticut , 5 5 North Ea g levilleRoad, S torrs, C T 06269-3060, U S A. E - m ail: nicholas.leadbeater@uconn.edu; Fax: +1 860 486 2981;Tel: + 1860 486 5076 ) irradiation, since the energy is interacting with the molecules ata very fast rate, the molecules do not have time to relax andthe heat generated can be, for short times, much greater thanthe overall recorded temperature of the bulk reaction mixture. Inessence, there will be sites of instantaneous localized superheatingwhere reactions will take place much faster than in the bulk. Thislocalized superheating can be especially marked when the reactionmixture contains highly polar reagents or catalysts. . Chemists often would use microwave irradiation as a last resort.heating reaction mixtures to high temperatures in sealed tubesin an attempt to make their reactions go. However, microwaveheating has a much more valuable role in the preparative chemist’sportfolio. It is possible to perform reactions at modest reactiontemperatures and still see great improvements in rate and yield.While sealed reaction vessels are one option, standard reflux andopen vessel chemistry can also benefit from microwave irradiationWhen performing synthesis using microwave heating, the usualprotocol is to heat the reaction mixture to a desired temperatureand then hold it there for a period of time. This is known astemperature control. During the initial heating stage a significantamount of microwave power is directed at the sample, but onceat temperature, the power drops such as to hold the reactionmixture at the desired temperature. By irradiating a reaction Jonathan Collins received a BS degree in biochemistry from the University of Florida in 2002. He leads the Bioscience Division at CEMCorporation in Matthews, NC. The Bioscience Division leads development of microwave applications in peptide synthesis, proteomics,genomics and other biomolecular fields. As well as developing apparatus for microwave chemistry applications, he and his group activelyresearch and publish in the area ofmicrowave-promoted peptide synthesis. Nicholas E. Leadbeater, a native ofthe United Kingdom, is an AssistantProfessor at the University of Connecticut. His research interests focusaround the use ofmicrowave heating as a tool for synthetic chemistry.Recent effort has been directed at organic chemistry in water, thescale-up of reactions, the use ofin-situ reaction monitoring as atool for synthesis and expanding the scope of microwave-promotedchemistry to new areas. He is a strong advocate of incorporatingundergraduate students into research and promoting clean chemistryandmodern technology in education. He is the co-author (with CynthiaMcGowan, Merrimack College MA) of“Clean, fast organic chemistry:Microwave-assisted laboratory experiments”. mixture with microwaves while simultaneously cooling the outervessel walls with compressed air or cryogenic fluid, it is possibleto irradiate the sample with significant microwave power duringthe whole period of the reaction. As well as potentially openingup avenues for new chemistry, it is possible to perform chemistryat lower bulk temperatures.5-7 As an example of this, in the Pd/Ccatalyzed Suzuki reaction of aryl chlorides with boronic acids inwater as a solvent, significant decomposition of the aryl chloridesubstrates occurs at the elevated temperatures required to effectthe coupling. By using microwave heating in conjunction withsimultaneous cooling it is possible to perform the coupling ata lower bulk temperature but in good yield.8 Another way tointroduce significant energy into a reaction mixture is to simplyirradiate the sample with a constant microwave power, allowing thetemperature to rise continually during the course of the reaction.This is known as power control. As the range of techniques for microwave heating has expanded,so have the areas in which it can have a profound impact. Oneof the emerging areas is the application of microwave heatingto biologically relevant processes. Examples of this are in thesynthesis of peptides, peptoids, oligopeptides and carbohydratesand in the field of proteomics. Here, we offer a perspective of thesetwo categories. Rather than provide an exhaustive survey, we havechosen to highlight the advantages of microwave heating in theseareas as well as discuss the potential of the technique to impactand transform the field in the future. Microwave-promoted synthesis of peptides Peptides are highly involved in many biochemical processesincluding cell-cell communication, metabolism, immune response,and reproduction. Additionally, peptides act as hormones andneurotransmitters in receptor-mediated signal transduction. Asthe role of peptides in many physiological and biochemicalprocesses has become more understood, so has interest in theirvalue as potential drug candidates. Peptides, when compared tosmall molecule drugs, have the advantage of higher potency andspecificity with fewer toxicological problems.’ A recent articlereported that there are more than 40 marketed peptides worldwide,around 270 peptides in clinical phase testing, and about 400 inadvanced preclinical phases.10,11 Obtaining peptides from natural sources can often be a verydifficult task. In tissue samples, desired peptides are often atvery low concentrations requiring highly sensitive assay methods.The availability and storage of natural tissue samples can alsolimit availability. While recombinant genetics has been the majorproduction tool for synthesis of proteins, this can be difficult, timeconsuming and laborious. Chemical synthesis of peptides allowsfor site-specific control of backbone and side chain modificationswith a specificity unavailable through recombinant strategies.Additionally, chemical synthesis allows for peptide sequences tobe synthesized not only rapidly, but also free from DNA impuritiesor endotoxins that may be present during recombinant synthesis.The combination of different chemical synthesis strategies hasbeen successful in producing small proteins of up to 200 aminoacids. Although chemical synthesis of peptides and proteins hasdeveloped greatly in the last several decades,it can be very timeconsuming and frequently requires the use of significant quantitiesof expensive reagents. In addition, in many cases synthesis can suffer from incomplete reactions that significantly reduce finalproduct purity. Chemical synthesis can be performed either bysolution phase, solid phase, or a combination of both. Solid-phasemethods predominate however, due to ease of purification at eachstep.2,13 The first example of microwave irradiation as a tool fonpeptide synthesis came in 1992. In their solid-phase synthesis ofthree test peptides, Yu and co-workers reported that microwaveheating offered a 2-4 fold reduction in amino acid couplingtime, especially when using side-chain hindered amino acids. Inaddition, no racemisation was observed.14 The reactions wereperformed using an un-modified domestic microwave apparatusand thus accurate temperature measurement was not possibleand also reproducibility from microwave to microwave provedan issue. While the results appeared promising, lack of properinstrumentation and concerns regarding acceleration of potentialside-reactions delayed further exploration. It was not until almosta decade later that the field began to develop in earnest. As modernmicrowave systems that generated a homogeneous microwave fieldand offered temperature control became available, new interest wasgenerated in peptide synthesis applications. a-Aminoisobutyricacid (Aib), was coupled with sterically hindered natural or non-coded amino acids, using conventional and microwave heatingmethods with either PyBOP/HOBt or HBTU/HOBt as couplingagents.15 In the microwave heating experiments, the reactionmixture was heated to 55 °C for 15 min followed by a further15 min at 60°C. Common activators such as HBTU and PyBOPhave been shown to be effective with microwave heating up to110°C. In the synthesis of a tripeptide (Fmoc-Thr-Val-Ile-NH,)and two dipeptides (Fmoc-Ala-Ile-NH, and Fmoc-Thr-Ile-NH,)(Scheme 1).16 The couplings were performed on a polystyreneresin using the Rink amide linker. Couplings of Fmoc-protectedamino acids were performed using microwave heating while theFmoc deprotection steps were conducted at room temperature.The microwave steps were performed in sealed vessels. The reactionmixtures were heated using a temperature control protocol to110°C and held at this temperature for 20 min. Significantpressure was generated during the course of the reactions dueto the volatile nature of the coupling agents used. The efficiency ofthe solid-phase methodology was however limited by the needto transfer between different reaction vessels to perform thecoupling and washing steps. The application of microwave heatingcan be expanded to both deprotection and coupling reactions.The 65-74ACP peptide, a standard peptide used to test syntheticmethods, was prepared reproducibly and in high purity using 2 mindeprotection and 3 min coupling steps and ending with a 10 mincleavage step. Schemele1 Microwave-promoted solid-phase synthesis of aa smalltripeptide. Ass attractive as the increased deprotection and couplingrates appeared, there was concern over possible enhancement of sequence dependent side reactions that are well-documentedconventionally. Aspartimide formation can occur during Fmocremoval with piperidine and can be a serious problem duringchain assembly. Nucleophilic attack by the nitrogen atom on theo-carboxy group of aspartic acid or aspargine on the side chainester or amide group, respectively results in generation of a 5-membered ring. This potential side reaction will accumulate dur-ing each successive deprotection step and can lead to substantiallyreduced purity. During the coupling reaction, formation of anactivated ester and concomitant increased acidity of the a-carbonproton can lead to racemisation. Conventionally this has beenproblematic for cysteine and histidine derivatives. Racemisationcan be difficult to detect because of difficulties with separation andidentical mass identification. While microwave peptide synthesishad shown no evidence of racemisation by HPLC analysiswith smaller peptides, there had not been a focus on cysteineand histidine containing peptides until recently when a groupattempted to prepare a 20 amino acid peptide containing His andCys residues using microwave heating. 18 The first synthesis in themicrowave at 70°C showed significant racemisation (3-5%).1 Thiswas suppressed by cooling the contents of the reaction vessel in anice bath before microwaving for a short period and then repeatingthis whole procedure a number of times. This allows for significantinput of microwave power while at the same time maintaining alow bulk temperature. The optimized conditions for making the20-mer involved deprotection steps of 3 pulses of 30 s at 100 W withcooling in between (Tmax =40°C) and coupling steps of 5 pulsesof 30 s at 50 W, again with cooling in between (Tmax =40°C). A nonapeptide has also been prepared using a similar“coolingbefore microwave irradiation”approach.2 The deprotection andcoupling reactions were performed in standard glass vials andthe washing steps in polypropylene syringes equipped with a fritand PTFE valve. Using a power control protocol, the maximumtemperature reached in the steps depended on whether the contentsof the reaction mixture were cooled to room temperature or to0°C prior to microwave irradiation as did the purity of the finalproduct. As with the previous synthesis of the 20- and 25-mers,cooling in ice prior to microwave irradiation proved optimal. While performing pre-cooling of reaction mixtures prior tomicrowave irradiation leads to peptides in high purity with littleracemisation, it is inconvenient and not practical either in anautomated reactor or when considering performing couplings inparallel or on a large scale. Addressing this problem has been thefocus of a recently published report.21 A model 20-mer peptidewas synthesized both at room temperature and using microwaveheating. The model peptide chosen for the study contained each ofthe natural 20 amino acids, but with a selectively placed C-terminalAsp-Gly segment to encourage maximum potential aspartimideformation (VYWTSPFMKLIHEQCNRADG-NH). At roomtemperature, although the racemisation levels were low, the crudeproduct purity was only 68% due to a number of deletions. Aninitial microwave-promoted synthesis of the 20-mer indicated thatthe sequence is susceptible to racemisation. Using deprotectionsteps of 30 s at 50 W followed by 180 s at 50 W (Tmax = 80°C)and a coupling step of 300 s at 40 W(Tmax=80°C) led tosignificant racemisation at His, Cys and Asp. Simply ensuring thatthe reaction mixture does not exceed 50°C when using a powercontrol microwave heating protocol proved effective in reducingracemisation of His and Cys residues and it is not necessary to pre-cool the reaction mixtures. A coupling protocol of 120 sat 0 W followed by 240 s at 40 W (Tmax = 50 °C) was used.Additionally, once Cys or His are incorporated into the peptidethey do not show any further increase in racemisation duringsubsequent chain extension steps, even when the reactions areperformed using the original conditions of heating to a maximumof 80°C. As an additional approach, couplings could be performedusing microwave heating with the exception of those involvingCys and His which instead are done at room temperature. Thiscombines the time savings of microwave irradiation for couplingof the majority of amino acids with the low racemisation possibleby coupling Cys and His conventionally. To limit aspartimideformation both incorporation of HOBt and using piperazinerather than the traditional piperidine as a reagent in deprotectionsteps proved effective. Solid-phase peptide synthesisers for use with conventionalheating have been around for many years and it was clearthat if microwave technology was to have a major impact onpeptide synthesis, an analogous automated synthesiser would berequired. This is because although the deprotection and couplingsteps could be accelerated using microwave heating, if manualtransfer between vessels for washing was required, any timesavings would be lost. While dedicated microwave synthesisers fororganic synthesis have been around for a few years, it was in 2003that the first automated equipment for peptide synthesis usingmicrowave heating became available."? The apparatus can performall steps necessary for the completely automated synthesis of apeptide including all deprotection, coupling, wash and peptide-resin cleavage steps. As an example, a 41 amino acid peptide hasbeen prepared using the apparatus in a total time of 31 h.2 The question arises as to why the microwave heating has sucha profound effect on the deprotection and coupling reactions tomake peptides, even at the relatively low microwave powers andbulk temperatures used.Optimal coupling conditions require afully solvated peptide-polymer matrix that allows for efficientreagent penetration. During the synthesis of difficult peptidesthe reaction matrix becomes partially inaccessible, typically 6-12residues into chain assembly.24,25 This could be attributed to theformation of secondary structures that result in poor solvationof the peptide-polymer matrix. As a peptide is built stepwise ona resin bead, it can form aggregates with itself or neighbouringchains as a result of hydrogen bonding between peptide backbones.One hypothesis is that irradiation with microwave energy leadsto de-aggregation of the peptide backbones thus allowing forreagents to reach the reaction sites at the end of growing chainsmore easily (Fig.1). The amide group of amino acids in a peptideshas two resonance forms (Scheme 2) thus leading to severalimportant properties, one of which is an unusually high dipolemoment of roughly 3.5 debye. When molecules that possess such adipole moment are exposed to microwave irradiation, the dipoles.try to align with the applied electric field. Since the electric fieldis oscillating, the dipoles constantly try to realign to follow thisAt 2.45 GHz, molecules have time to align with the electric field Scheme 2 Resonance forms of an amide group in an amino acid. a b Fig.1 A peptide backbone (a) before and (b) during microwave irradiation. but not to follow the oscillating field exactly. This continual re-orientation of the molecules is one of the accepted mechanisms bywhich microwaves lead to localised heating of reaction mixtures.2In a polypeptide, this movement could quite feasibly lead tode-aggregation of the peptide backbone as well as to localisedsuperheating which can have an acceleratory effect on the reactionrate for deprotection and coupling steps. To show the effectiveness of microwave heating in the prepara-tion of longer peptide sequences a β-amyloid, the main constituentof the fibrillar aggregate responsible for Alzheimer’s disease, hasbeen prepared.27 The 42 amino acid peptide could be preparedin 69% purity in 19 h using the automated microwave peptide.synthesiser. Microwave heating has been used to prepare 14-helical B-peptides.28 Initial studies focused on the optimisation of theconditions required to prepare single peptides.2 Hexamer 1 wasprepared manually both conventionally and also in a monomodemicrowave apparatus. When using conventional methodology,although the penta-B-peptide precursor was >95% pure, hexamer1 was only 55% pure with significant quantities (33%) of theunreacted pentamer as well as some of the Fmoc-protectedhexamer being present. Using controlled microwave heating, 1 wasobtained in 80% purity with only 5% of the unreacted pentamerand none of the Fmoc-protected hexamer being observed.Studieswere extended to the deca-B-peptide 2. However this was obtainedin only 57% purity, indicating the challenge of coupling extraresidues to 1. A solution to the problem was to use a solution of LiCl in DMF in the coupling protocol. Salt additives are knownto alleviate, at least in part, the problems of aggregation and/orfolding of resin-bound a-peptides as they increase in length.Thus, in this case, the effects of microwave heating alone maynot be enough to disaggregate the p-peptide backbone. With themodified reaction conditions, 2 could be prepared in 88% purityand 81% yield. Returning to the synthesis of 1 and using themodified conditions, the already high purity could be increasedeven more (94%). As well as de-aggregating the peptide, the saltadditive could also enhance the ionic conduction mechanism forconversion of microwave energy into heat in the reaction mixture. Moving to a multimode microwave apparatus, the same grouphave prepared a library of 14-helical β-peptides.31 First, conditionsfor the preparation of 1 in the multimode microwave apparatuswere re-optimised. Then, to confirm the homogeneity of heatingand to optimise stirring in a 96-well plate, 1 was prepared in 26different wells scattered across the plate. A library of peptides wasthen prepared. Some variation in product purity was observed.While this could be attributed to inhomogeneity of heating acrossall the wells, since the differences in purity were no more than 10%,it could be as much dependent on the amino acid sequence as thelocation in the 96-well plate Peptoids can be prepared efficiently using microwave heating.Peptoids differ from peptides in that the side chain is connectedto the amide nitrogen rather than the a carbon atom and areknown as oligo(N-alkyl) glycines. Peptoids exhibit enhancedstability towards proteolysis relative to a-peptides." They also find applications as biological probes and in drug discovery.33.34In preparing peptoids, each building block is added in a two-stepstrategy known as the“sub-monomer”route (Scheme3).35 Firstlythe amine function of the existing chain is acylated by the additionof bromoacetic acid and N,N'-diisopropyl carbodiimide (DIC)and then nucleophilic replacement of bromide with a primaryamine. The commercial availability of a wide range of structurallydiverse primary amines means that large, diverse peptoid librariescan be made easily and cheaply, often without the need forprotecting groups. However, the procedure is slow. Starting fromRink MBHA amine resin, the solid-phase synthesis of a 9-mercould take 20-32 h. Using a domestic microwave oven, this hasbeen reduced to about 3 h.36 The reactions were performed ina 1000 W microwave oven with the power set at 10%. Solutionswere irradiated for 15 s, gently agitated and then irradiated fora further 15 s. Under these conditions, the bulk temperature ofthe reaction mixture was found not to exceed 35 °C as determinedusing a thermometer after the second 15 s irradiation. This 2×15 sirradiation protocol was used for both the acylation and bromidesubstitution reactions. Yields and purities were comparable tothose obtained when performing the chemistry conventionally at37°C (45 min for acylation, 1 h for bromide substitution). Scheme 3 Synthesis of peptoids using the sub-monomer route. Following this work, a protocol has been developed for thepreparation of peptoids in a scientific microwave apparatus.As well as offering a more reproducible methodology, someinteresting observations were made. In moving from domesticto scientific microwave equipment, simply repeating the reactionsusing the same conditions (100 W for 30 s) gave peptoids thatwere some 10-50% lower in purity. In attempts to determine theorigins of this difference, it was discovered that in the case ofunhindered primary amines no microwave irradiation was requiredin order to obtain the corresponding peptoids in high purity. Thusneither microwave heating nor the lengthy conventional protocolis necessary, the reactions being complete within approximately1 min at room temperature. With electronically deactivatedbenzylamines, microwave heating was found to have a positiveeffect. The optimal conditions for the acylation step involvedirradiation for 30 s and heating to 35°Cusing temperature control,and for the bromide displacement, irradiation for 90 s and heatingto 95 °C again using temperature control. More recently, microwave heating has been found to be useful forgenerating a poly-cationic peptiod and its conjugation to a 13-merpeptide. In an extension to the work, microwave heating has alsobeen used to label the peptides with a variety of fluorophores andquenchers.38 Since more than half of all proteins carry carbohydrate side-chains, the synthesis and study of glycopeptides has becomean important area of research.39.40 The chemical synthesis ofglycopeptides has particular advantages. Glycoprotein samplestraditionally obtained from biological sources are very complexand, as a result, little is known about how the glycan chainsspecifically modulate stability and activity. One synthetic route toglycopeptides involves the use of β-glycosylamines as intermedi-ates, these then being reacted with a suitably protected amino acidor polypeptide side chain." The β-glycosylamines can be prepareddirectly from a fully deprotected sugar by treatment with 40-50equivalents of ammonium bicarbonate at room temperature for6 days, this being known as the Kochetkov reaction." By usingmicrowave heating, this reaction can be performed in 90 min at40°C (Scheme 4).43 Key to the success of the reaction is use ofDMSO as a solvent. It is also possible to reduce the quantity ofammonium bicarbonate required to 5 equivalents. A microwavepower of 10 W is used and a temperature control protocol used.If the reaction temperature is increased to 60°C, significantdimerisation is observed. The crude glycosylamines obtained weresubsequently used for the preparation of glycoamino acid buildingblocks. Scheme 4 Microwave-promoted synthesis of β-glycosylamines. Microwave heating has also been used for the coupling ofsterically hindered, glycosylated amino acid building blocks onsolid supports.44,45 Amino acid building blocks 3 and 4 wereused in the building of a 20 amino acid protein, 5, knownas MUC1. This five O-glycan containing glycopeptide has anantigenic structure and is found on the surfaces of epithelial cells ina variety oftissues.46 The solid-phase synthesis was performed onboth Tentagel and poly(ethylene glycol) poly(dimethylacrylamide)copolymer (PEGA) supports functionalised with Rink amidelinker. Reactions were performed at 50 °C in a monomodemicrowave apparatus using a temperature control protocol as wellas conventionally at 50 °C and room temperature. Starting withthe Tentagel support, when coupling regular Fmoc-amino acids areaction time of 10 min was used whereas for couplings involving3 and 4, the time was extended to 20 min. Deprotection steps wereperformed in 3 min. The whole procedure for building the 20-merwas undertaken manually, taking 7 h in the case of the microwavereactions and the conventional control at 50 C. For the couplingsat room temperature, reaction times were significantly extended(2 h for Fmoc amino acid couplings, 20 h in the case of 3 and 4and 20 min for Fmoc deprotection steps). As a result, the wholeprocess took approximately 99 h. The overall yield of the desired20-mer in the microwave protocol was significantly higher thanwhen using conventional heating but comparable to that obtainedat room temperature. Changing to the PEGA resin improved theyield of the final glycopeptide. The PEGA resin may permit thepermeation both of reagents and steric building blocks 3 and 4into the porous surfaces of the polymer particles. The 20-mer was OAc AcO· AcO- OAc NHAc OHAcO-AcO-OAc AcHNH-Pro—Pro—Ala-His-Gly-Val-Thr-Ser-Ala—Pro—Asp-Thr-Arg—Pro—Ala—Pro—Gly-SerThr-Alaa—QNHAc OAc NHAc-OAcOAcOH AcHN OAc -OAc OAc 5 OAc elaborated further by enzymatic sugar elongation to give a rangeof morecomplicated glycopeptides. Microwave heating as a tool for proteomics Proteomics is broadly defined as the large-scale study of proteins,particularly their structures and functions.47,48 To obtain detailedstructural information, proteins are selectively cleaved into smallerpolypeptide fragments by controlled chemical reactions or enzy-matic digestion. The resulting mixtures can then be analysed byvarious mass spectroscopic techniques and, from this, the structureof the original protein determined. Identification of the amino acid at the C-terminus of proteinsis possible using the Akabori reaction, devised over half a centuryago. It involves heating the protein with anhydrous hydrazineunder reflux at 125°C for several hours. The amino acid at theC-terminus of the peptide is liberated and can be distinguishedfrom the remaining amino acid residues that have been convertedto hydrazides. In addition, hydrazinolysis provides quantitativeinformation for the presence of amino acid residues containingguanidino, β-mercapto, carboxy, or carboamido groups. Thereaction has been performed in a domestic microwave, the timeto reach completion being reduced to 3-5 min. The dipeptideTrp-Phe, tripeptide Tyr-Gly-Gly, tetrapeptide Pro-Phe-Gly-Lys,heptapeptide Ala-Pro-Arg-Leu-Arg-Phe-Tyr, and an N-terminalblocked tripeptide (N-acetyl-Met-Leu-Phe) have all been used astest substrates. When using enzymic methodologies, digestion times depend onthe nature of the proteins and can vary from hours to days. It is necessary to ensure that sufficient quantities of the peptides aregenerated such that the detection limit of the analytical techniquesis surpassed.Trypsin is the most commonly used enzyme becauseit specifically hydrolyses peptide bonds at the carboxyl side oflysine and arginine residues, except when either is followed byproline. Unmodified trypsin is subject to proteolysis, generatingfragments that can interfere with protein sequencing or peptideanalysis. As a result, trypsin modified by reductive methylationis often used, this treatment rendering it resistant to proteolyticdigestion. The combination of trypsin digestion with controlledmicrowave heating has attracted attention. Trypsin digestion of bovine cytochrome c, bovine ubiquitin,horse heart myoglobin, modified chicken egg lysozyme andrecombinant human interferon a-2b (rh-IFN a-2b) has beenperformed in a scientific monomode microwave apparatus. Thereactions were performed on a 100-350 uL scale. The digestionwas optimised at 60 °C for 10 min. Additionally, no non-specific cleavage was detected using the microwave approach.A comparison of conventional and microwave heating in thedigestion of cytochrome c showed that microwave irradiation for10 min produced similar results to the classic method of 6 h ofdigestion at 37 °C. The beneficial effect of microwave irradiationwas confirmed by performing the digestion of cytochrome c in12 min under microwave and conventional heating. In the case ofmicrowave irradiation, after this time approximately 90% sequencecoverage was possible whereas there were no hydrolysis productsin the conventional experiment. In the absence of the protease,cytochrome c remains intact after 20 min irradiation, showing thatthe microwave energy only enhances the enzymatic digestion of the protein and does not itself induce degradation. Similar shorteningof the digestion time has been found when using a domesticmicrowave and bovine serum albumin and human urinary proteinas substrates. The digestion of rh-IFN a-2b was repeated at different tem-peratures and rapid elevation of the solution to above 60°C wasfound to enhance the process. In both cases the percentage ofdigestion increased rapidly over the first 5 min before levellingoff at approximately 70% at 10 min. In an attempt to mimicconventionally the temperature profile seen in the microwaveexperiments, the same digestion was performed using a metal blockpre-heated to 60°C. The rate of enzymic cleavage was found tobe essentially identical suggesting that, at least in part, the rapidincrease in temperature is responsible for the rate of accelerationseen upon microwave irradiation. Using more accurate temperature measurements and con-trol, the microwave-mediated digestion of glycated haemoglobin(HbA1c) has been studied.3 Using trypsin modified by reductivemethylation as the proteolytic enzyme for digestion, the optimumconditions involved controlled microwave heating to 50 °C andholding at this temperature until a total time of 20 min had elapsedwith an enzyme-to-protein ratio of 1 : 100. Under these conditions,the digestion efficiency is about 20% greater than that observedusing conventional conditions for 18 h. Changing the enzyme fromtrypsin to Glu-C has a significantly negative effect on digestionefficiency. The optimum temperature for Glu-C was found to be40°C but, even at this zenith, the efficiency was significantly lowerthan under conventional conditions at room temperature. Thedifference in enzyme activity could be attributed to several factors.Firstly, Glu-C was used at temperatures significantly higher thanthe manufacturer’s recommendation of 25 °C. Also, while it ispossible to modify trypsin to make it less susceptible to proteolyticdigestion, the same is not true of Glu-C. However, since analysis ofthe Glu-C digest showed no evidence for proteins originating fromthe enzyme, it is more likely that thermal deactivation is the majorcause of the loss of activity as opposed to autolysis of the enzyme. Digestions are traditionally performed in standard buffersolutions. The effect of solvent on the efficiency of microwave-promoted trypsin digestion has been studied using myoglobin,cytochrome c, lysozyme and ubiquitin as substrates.s2 The effi-ciencies and sequence coverages increased when acetonitrile wasadded to the reaction mixture. If methanol is used as an additive, asthe quantity increases so the enzyme activity decreases indicatingthat it is being deactivated. This is not surprising given the factthat methanol inhibits many enzyme-catalysed processes. It is also possible to use microwave heating to perform digestson substrates separated on gels and results suggest that thissignificantly improves peptide recovery compared to standard in-gel digestion protocols.51,52,54 The ability to be able to performin-gel digests means that the technology can be in conjunctionwith separation techniques for analysis of protein mixtures.Five proteins, including lysozyme, chicken egg albumin, bovinealbumin, conalbumin, and ribonuclease have been separated by gelelectrophoresis, stained, cut out and digested with trypsin solutioneither in a microwave for 5 min or incubated for 5 min or 16 hat 37°C. Using microwave irradiation resulted in more matchedfragments than either of the traditional methods for all the proteinsexcept conalbumin; in this case the number of matched fragmentsis slightly lower but close to the 16 h traditional method. Other Table 11Comparative dipole moments5 Molecule Dipole moment (debye) Water Peptide bond Myoglobin 170 Horse serum albumin 380 Horse carboxy haemoglobin 480 studies show similar results.For example, with both bovine serumalbumin and yeast lysate the number of proteins identified with anin-gel microwave methodology was either the same or better thanthat from conventional studies.52 Again the question arises as to why the microwave heating hassuch a profound effect on the rate ofenzymatic digestion. Together,a-helixes and B-conformations compose a major portion of the sec-ondary structure of large proteins. In the o-helix, the polypeptidebackbone is tightly wound around the molecule axis with eachamino acid side-chain pointing outwards and downwards fromthe backbone. The a-helix shows highly optimised use of internalhydrogen bonding and creates a stacking of peptide bond dipolesthat are added across the hydrogen bonds in the helix, this leadingto a large net dipole from one end of the helix to the other. Themagnitude of these dipoles is illustrated in Table 1. The presenceof a large net dipole moment across a-helices may be responsible,at least in part, for increased digestion rates of certain proteinsupon microwave irradiation. If the microwave energy interacts withthe dipole of the a-helix, perturbation of the three-dimensionalstructure of the protein may result (Fig.2). This could facilitatedigestion of previously enclosed areas of the protein. Fig.22 DDipole moment across an a-helix and interaction with microwaveradiation. Microwave heating can also be used for determination of aminoacid content (but not structural information) by means of vapour-phase protein hydrolysis using 6 M HCl in a sealed tube.56 Aparticular advantage of the microwave-promoted hydrolysis is thatit reduces the time required for cleavage of difficult to hydrolysehydrophobic peptide linkages without excessive degradation of thelabile amino acids, serine and threonine. In a recent development,apparatus specifically designed for microwave-promoted hydroly-sis has become available. The 45 mLvapour-phase hydrolysis vesselallows processing of up to ten 100-300 pL HPLC autosamplervials at one time and is connected to vacuum and nitrogen sources.The sealed hydrolysis vessel is alternately evacuated and purged with nitrogen. The hydrolysis is then performed under inert,anaerobic conditions to prevent oxidative degradation of aminoacids. Using this, hydrolyses on the picomole to nanomole scaleare possible. Microwave heating can be used as a tool for deglycosylationof antibodies.7Many biotechnological products consist of an-tibodies raised against an oncological, auto-immunological orother antigen of interest. Characterisation of these antibodiesis important, but the fact that they are heavily glycosylatedmeans that the task of measuring the molecular weight of theintact protein is complicated. By deglycosylation, it is possibleto facilitate accurate molecular weight verification. A commonmethod for achieving this is to use an enzymic cleavage. Peptide-N-glycosidase F (PNGase F) is one of the most widely used enzymesfor the deglycosylation of glycoproteins. The enzyme releasesasparagine-linked (N-linked) oligosaccharides from glycoproteinsand glycopeptides. With controlled microwave heating it is possibleto perform deglycosylation reactions using PNGase F in between10 min-1 h depending on the substrate. This compares to atime of up to 2 days when performing the same transformationsconventionally. The reactions were performed in a monomodeapparatus using just 1 W microwave power. The optimum reactiontemperature was 37-45 °C above which recovery of the protein atthe end of the cycle drops significantly. Microwave heating as a tool for other biochemicalapplications Microwave heating has found uses in a number of other biochemi-cal processes. To show the scope, we have selected some examples. Microwave-promoted catalysis oforganic transformations usingenzymes has been the subject of a number of studies, mainly usingdomestic microwave ovens.58 While an apparent rate enhancementhas been reported, more control experiments are required beforea definite conclusion can be drawn. Controlled microwave irra-diation has been used to accelerate by at least 15-fold metal-catalyzed oxidation reactions that site-specifically oxidize theamino acids bound to copper in Cu/Zn superoxide dismutase.When combined with mass spectrometry, these reactions providea sensitive method for determining Cu-protein binding sites. Themaximum microwave power suitable for maintaining the structuralintegrity of the protein can be determined readily by measuringthe oxidation extent of different peptide fragments as a functionof power. DNA amplification by polymerase chain reactions (PCR) isa very powerful process, finding applications in medical andbiological research labs for a variety of tasks such as the detectionof hereditary diseases, the identification of genetic fingerprints, thediagnosis of infectious diseases, the cloning of genes and DNAcomputing.60-62 The PCR process is conventionally carried out ina thermal cycler using a DNA polymerase. The most common isTaq polymerase. The application of microwave heating to PCRhas been studied both to see how the process can be facilitatedand also probe the effects of heating Taq polymerase in manycycles.63,64 Using controlled microwave heating, it is possible toreduce the cycle time by approximately half. This is due mainlyto the fact that it is possible to reach the target temperatures veryrapidly and then hold it there easily. As a result, incubation times can be shortened over the conventional counterparts since, inthe case of the latter, time for equalisation of the temperatureis required. Also, because the heating is on a localized level whenusing microwave irradiation, it is possible to perform PCR on themL scale. Conventionally, the slow distribution of heat togetherwith the importance of short process times and reproducibilitylimits the volume for most reactions to 0.2 mL. Using microwaveheating, PCR on the 2.5 mL and 15 mL scales has been performedrapidly with very high efficiency. The sequence to be amplified wasa 53 bp fragment from human chromosome 13. A total of 33 cycleswas performed. By combining the use of metal-enhanced fluorescence (a nearfield effect that can significantly enhance fluorescence signatures)with low power microwave heating, the sensitivity ofsurface assaysin a model protein avidin-biotin assay could be greatly increasedas well as being essentially completed within a few seconds.A greater than 5-fold fluorescence enhancement coupled withan approximate 90-fold increase in assay kinetics was observed.This technology has the potential to impact high throughputfluorescence-based processes, such as in biology, drug discoveryand general compound screening. In another enabling technology,the power of combining cleavable isotope-coded affinity tags(ICAT) and microwave heating can offer an efficient, highthroughput analysis of proteins. Summary Application of microwave energy for peptide synthesis hasshown advantages in terms both of higher purity and shortersynthesis time. With two separate chemical reactions requiredfor addition of each amino acid, the benefit of microwave isaccumulated at each cycle. Synthesis of other types of polymerssuch as oligonucleotides and carbohydrates should offer similarbenefits. Phosphoramidite chemistry has become the preferredsynthesis technique of oligonucleotides. While DNA synthesis isfast and efficient conventionally (30 s coupling), RNA synthesis ismore difficult. In comparison to deoxyribose phosphoramidities,the ribose phosphoramidites associated with RNA contain anextra 2'-hydroxyl group that requires additional protection duringsynthesis. The commonly used protecting groups2'-O-(tert-butyl)-dimethylsilyl (TBDMS) and 2'-0-[(triisopropylsilyl)-oxy]-methyl(TOM) can interfere with the coupling reaction requiring routine12-15 minute reaction times with standard tetrazole activation.Use of alternative activators such as 5-ethylthio-1H-tetrazole(ETT) and 5-benzylthio-1H-tetrazole (BTT) has shown decreasedcoupling times, but their increased acidity has been associatedwith premature deprotection of trityl groups leading to unwanteddimerisation. Microwave represents a strategy for potentiallydecreasing RNA coupling times to less than a minute even whileusing standard tetrazole activation. Removal of the 2'-protectinggroup of TBDMS or TOM is a slow process that is critical forhigh purity RNA. Conventionally, this process requires around3 h and is a potential area where microwave synthesis couldprovide benefits. Carbohydrates have many biological roles including energystorage, metabolism, and bioreceptors in cell-to-cell communi-cation. However, research of carbohydrates has been limited dueto difficulty of synthesis. Development of solid phase synthesisof oligosaccharides (SOS) represents an area of active research to overcome this problem. However, generation of syntheticoligosaccharides represents a major challenge due to the fact thatthey are branched rather than linear, monosaccharide units canbe connected by a or β linkages, and multiple selective protect-ing strategies are required. Attempts at coupling monosaccha-ride units are also difficult due to steric hindrance associated withthe monosaccharide units. While development of an orthogonalprotecting strategy for SOS represents a major current challenge,microwave represents a valuable tool that can be applied to assistthe rapidly developing SOS methods. A proteome is thought to be an order of magnitude more com-plex than the genome itself due to post-translational mechanisms,genes coding for multiple proteins, and proteins assuming multipleforms. For this reason throughput is a major focus in proteomicsand enzymatic digestion is often conventionally performed in96-well formats. While enzymatic digestion has shown benefit withmicrowave energy, its application has typically been limited tosingle samples. A high throughput format is currently an issuefor microwave instrumentation as the individual wells do not heatevenly in larger multimode cavities and do not fit into smaller singlemode cavities. Research focused on development of a new cavityto heat a 96-well plate uniformly has proved challenging due tothe small sample sizes used and the need for accurate temperaturemonitoring. Development of a 96-well plate microwave systemshould make a significant impact in improving throughput andsequence coverage for proteomics. In conclusion, microwave chemistry has shown its scope is notlimited only to synthetic organic chemistry, but also includes manybiological applications. In many cases, comparison of conven-tional and microwave methodologies has shown that peptidescan be prepared in higher yield and purity using microwaveirradiation.21,29,37,38 Due to the large net dipole moment associatedwith peptides, proteins, and other biomolecules, microwave energyoffers a unique tool in the bioscience field. Through dipole rotationand ionic conduction. controlled microwave irradiation can beused to transfer significant amounts of energy into biosystemsfor enhancing a wide variety of processes. As the bioscience fieldcontinues to expand so the application of microwave will grow inparallel. References ( 1 A number of books on m i crowave-promoted synthesis have beenpublished recently: (a) Microwaves in Organic Synthesis, ed . A . Loupy,Wiley-VCH , W e inheim, 20 0 6; (b) C . O . K a ppe and A. Sta d ler, Mi - crowaves in Organic and Medicinal Chemistry, Wiley-VCH,Weinhiem,2005; ( c) Microwave-Assisted Organic Synthesis, e d . P . Lidstrom andJ. P. Tierney, Blackwell , Oxford, 2005; (d) Microwaves in OrganicSynthesis, ed. A. L oupy, Wiley-VCH, Weinheim, 2002;(e) B. L. Hayes,Microwave Synthesis: Chemistry at the SpeedofLight, CEM Pu b lishing, M atthews NC, 2002. ) ( 2 F or r ecent reviews see: (a ) C . O . Kappe, Angew. Chem., I nt. Ed., 2004, 43, 6250; (b) M. Larhed, C. M oberg and A. Hallberg, Acc. Chem.Res., 2002, 35, 717; ( c ) A. Lew, P . O. Krutzik, M. E. Hart a n d A. R.Chamberlain,J. Co m b. Chem., 2002,4,95;(d) P. Li d s t rom, J. P. Tierney,B. Wathey and J.Westman, Tetrahedron, 2001,57,9225. ) ( 3 R. Gedye, F . Smith, K. Westaway, H. Ali and L. B aldisera, Tetrahedron Lett.,1986,27,279. ) ( 4 R. J . Giguere, T. Bray, S . M. Duncan and G . M a jetich, T e trahedronLett.,1986,27,4945. ) ( 5 J.J. C hen and S. V. Deshpande, Tetrahedron Lett. , 2003,44,8873. ) ( 6 C . E . Humphrey, M . A. M. Easson, J.P . T i erney and N . J .T u rner, Org. Lett.,2003,5,849. ) 7 N. E. Leadbeater, S. J. Pillsbury, E. Shanahan and V. A. Williams,Tetrahedron, 2005,61,3565. 8 R. K. Arvela and N. E. Leadbeater, Org. Lett., 2005, 7,2101. 9 Drug Discovery and Development, ed. M. S. Chorghade, Wiley, NewJersey, 2006. 10 Chemical and Engineering News, March 14, 2005, vol. 83, No. 11, pp.17-24. 11 Natural peptides such as insulin, vancomycin, oxytocin and cy-closporine, and synthetically produced ones such as Fuzeon (enfuvir-tide) and Integrilin (eptifibatide) are among the approved peptide-baseddrugs. 12 For a practical introduction see: E. Atherton and R. C. Sheppard,Solid Phase Peptide Synthesis: A Practical Approach,Oxford UniversityPress,Oxford,1989. 13 For the initial reports of solid-phase peptide synthesis see: R. B.Merrifield, J. Am. Chem. Soc., 1963,85,2149. ( . 14 H.-M. Yu, S.-T. C h en a nd K.-T. Wang, J. Org. Chem., 1 9 92,57 , 4781. ) 15 V. Santagada, F. Fiorino, E. Perissutti, B. Severino, V. De Filippis, B.Vivenzio and G. Caliendo, Tetrahedron Lett., 2001,42,5171. ( 1 6 M . Erdelyi and A. Gogoll, Sy n thesis, 20 0 2, 15 9 2. ) ( 1 7 J . M. Collins and M. J. Collins, in Mic r owaves in O rganic Synthesis,ed. ) ( A . L oupy, Wiley-VCH, We i nheim, 2006, ch. 20, pp . 898-93 0 . ) 18 A. Rybka and H. G. Frank, poster presentation at the 19th AmericanPeptide Symposium, San Diego, CA, 2005. 19 The observation that racemisation was not a problem in the initial 1992report 4 of microwave-heating in peptide synthesis or in the subsequentreports at elevated temperatures15,16 could be attributed to the fact thatnone of these troublesome residues were constituent parts of the peptideproducts. 20 B. Bacsa, B. Desai, G. Dibo and C. O. Kappe, J. Pept. Sci., 2006, 12,633. 21 S. A. Palasek, Z. J. Cox and J. M. Collins, J. Pept. Sci., 2006, 12, inpress. ( 22 C E M Liberty system. See: www.cempeptides.com. ) ( 23 R evi e wed i n : S . Kutter, Wirtschaftswoche,2005,8, 69. ) ( 2 4 J . P. Tam and Y. A. Lu, J . Am. Chem. Soc., 1995,1 1 7, 1 2058. ) ( 25 R . M errifield a nd V. L ittau, in Peptides, ed. E . Bricas, North HollandPublishers, Amsterdam, 1 968. ) 26 For an introduction to the physical chemistry concepts of microwaveheating see: (a) D. Stuerga, in Microwaves in Organic Chemistry, ed.A. Loupy, Wiley-VCH, Weinheim, 2006, ch. 1; (b)D. M. P. Mingos, inMicrowave-AssistedOrganic Synthesis, ed.P. Lidstrom and J. P.TierneyBlackwell, Oxford, 2004, ch. 1; (c) C. Gabriel, S. Gabriel, E. H. Grant,B. S. Halstead and D. M. P. Mingos, Chem. Soc. Rev., 1998, 27,213. 27 S. A. Palasek, Z. J. Cox and J. M. Collins, presentation at the 16thInternational Conference on Organic Synthesis, Merida, Mexico, 2006. 28 B-Peptides consist of β amino acids, which have their amino groupbonded to the B carbon rather than the a carbon as in the 20 standardbiological amino acids. 29 J. K. Murray and S. H. Gellman, Org. Lett.,2005,7,1517. 30 See for example: J. C. Hendrix, K. J. Halverson, J. T. Jarrett and P. T.Lansbury,J. Org. Chem.,1990,55,4517. 31J. K. Murray and S. H. Gellman, J. Comb. Chem., 2006,8,58. ( 32 S . M. Miller, R. J. Simon, S. Ng, R . N . Z uckermann, J. M. Kerr andW. H. Moos, B ioorg. Med. Chem. Lett . ,1994,4,2657. ) 33 P. G. Alluri, M. M. Reddy, K. Bachhawat-Sikder, H.J. Olivos and T.Kodadek, J. Am. Chem. Soc.,2003,125,13995. 34 J. A. Patch, K. Kirshenbaum, S. L. Seurynck, R. N. Zuckermann andA. E. Barron, in Pseudopeptides in Drug Development, ed. P. E. Nielsen,Wiley-VCH, Weinheim, 2004, pp. 1-31. 35 R. N. Zuckermann, J.M. Herr, S. B. H. Kent and W. H. Moos, J. Am.Chem. Soc.,1992,114,10646. 36 H. J. Olivos, P. G. Alluri, M. M. Reddy, D. Salony and T. Kodadek,Org. Lett.,2002,4,4057. 37 B. C. Gorske, S. A. Jewell, E. J. Guerard and H. E. Blackwell, Org.Lett.,2005,7,1521. 38 M. A. Fara, J. J. Diaz-Mochon and M. Bradley, Tetrahedron Lett.,2006,47,1011. 39 Glycopeptides and Glycoproteins: Synthesis, Structure, and Applica-tion, Topics in Current Chemistry, ed. V. Wittmann, Springer, Berlin,2006,vol.267. 40 Glycopeptides and Related Compounds: Synthesis, Analysis, and Appli-cations,ed.D. G. Large and C.D. Warren, Marcel Dekker, New York,1997. ( 41 S ee f or example: M. W agner, S. Dziadek and H. Kunz, C h em.-Eu r . J, 2 003,9, 6 018; S. T. Cohen-Anisfeld and P . T. Lansbury, J . Am. Chem.Soc.,1993,115,10531. ) ( 42 L . L i khosherstov,O. Novikova,V. A. Derveiskaja and N. K . K o chetkov,Carbohydr. R es., 1995, 6, 3 16. ) ( 43 M . Bejugam and S. L. Flitsch, Org. Lett., 2004, 6,4001. ) ( 44 T. Matsushita, H. Hinou, M. K urogochi, H. S himizu and S . -I.Nishimura, Org. L ett., 2005, 7 , 8 77. ) ( 45 T . Matsushita, H. Hinou, M. F u moto, M. Kurogochi, N. Fu j itani, H. Shimizu and S.-I. Nishimura, J. Org. C h em., 2 006, 7 1, 3051. ) ( 46 F .-G. H anisch and S. M oller, Glyc o biology , 2000, 1 0,439. ) ( 47 R . Twyman, Principles of Proteomics, Wiley-VCH, Ta y lor and Francis, London, 2004. ) ( 48 R . W estermeier a n d T. N a ven, Pr o teomics i n Pr a ctice: A La b oratory Manual of Proteome Analysis,Wiley- V CH , Weinheim, 2002. ) ( 49 S . Akabori, K .O h no and K. N ar i ta, Bull. Ch e m. So c . Jp n ., 19 5 2, 25, 214. ) ( 50 A . K. Bose, Y. H. Ing, N. Lavlinskaia, C. Sareen, B. N . Pramanik,P. L. B artner, Y.-H. Liu and L. Heimark, J. A m. Soc. Mass Spectrom., 2002, 13,839. ) ( 51 B . N. P ramanik, U. A. M i rza, Y.H. Ing, Y.-H. Liu, P. L. Bartner, P . C.Weber and A . K . Bose, Protein S c i., 2002, 1 1,2676. ) ( 52 W. Sun, S. Gao,L. Wang, Y. Chen, S . Wu, X. Wang, D. Z heng and Y. Gao,Mol. Cell. Proteomics , 2 006,5,769. ) ( 53 H. W. Vesper, L . Mi, A. Enada and G. L . Myers,Rapid Commun. Ma s s Spectrom. , 2005, 1 9,2865. ) 54 H.-F. Juan, S.-C. Chang, H.-C. Huang and S.-T. Chen, Proteomics,2005,5,840. 55 From R. Pethig, in Protein-Solvent Interactions, ed. R. B. Gregory,Marcel Dekker, New York, 1994, pp. 265-288. 56 S. T. Chen, S. H. Chiou and K. T. Wang, J. Chin. Chem. Soc.,1991,38,85. 57 J. Lill,W. Sandoval, H. Raab,F. Arellano,R. Vandlen and D. Arnott,poster at the 54th ASMA Conference on Mass Spectrometry, SeattleWA, 2006. 58 For a review see: I. Roy and M. N. Gupta, Curr. Sci., 2003, 85,1685. ( 59 J . D. Bridgewater and R. W. Vachet, Anal. Chem., 2005,7 7 ,4649. ) 60 M. J. McPherson and S. G. Moller, PCR (The Basics), Taylor andFrancis, London, 2006. 61 PCR Primer: A Laboratory Manual, ed. C. W. Dieffenbach andG. S. Dveksler, Cold Spring Harbor Laboratory Press, New York,2003. 62 http://en.wikipedia.org/wiki/Polymerase_chain_reaction. 63 C. Fermer, P. Nilsson and M. Larhed, Eur. J. Pharm. Sci., 2003, 18,129. 64 K. Orrling, P. Nilsson, M. Gullberg and M. Larhed, Chem. Commun.,2004,790. 65 K. Aslan,P. Holley and C. D. Geddes,J. Immunol. Methods, 2006,312,137. 66 T.M. Pekar, M.-L. Nguyen, J.Rutherford, D. Innamorati, J. Bonapaceand J. Pirro, personal communication. his journal is O The Royal Society of Chemistry rg. Biomol.Chem.,
关闭-
1/10

-
2/10
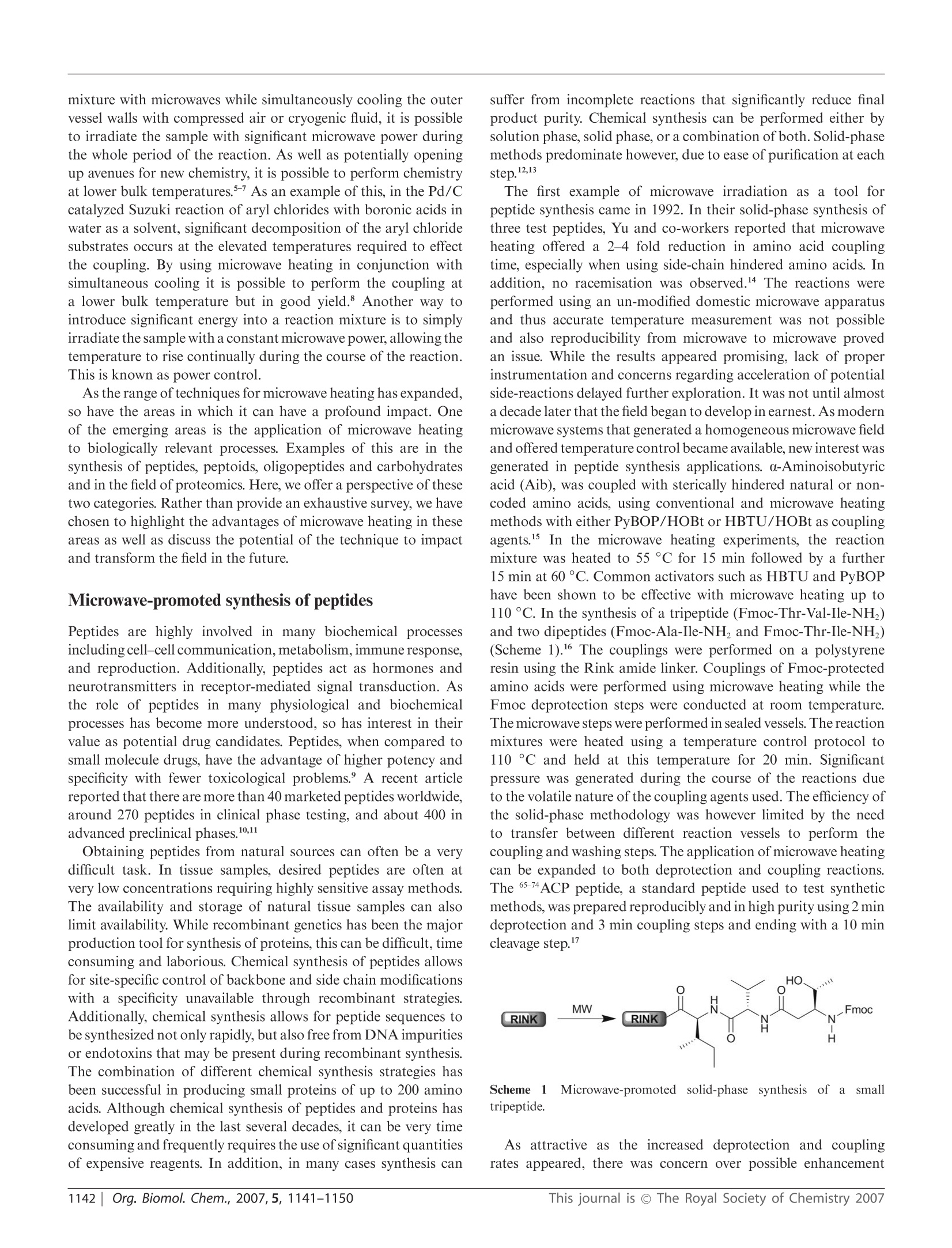
还剩8页未读,是否继续阅读?
继续免费阅读全文产品配置单
培安有限公司为您提供《生命科学研究中微波能量工具检测方案(多肽合成仪)》,该方案主要用于其他中微波能量工具检测,参考标准《暂无》,《生命科学研究中微波能量工具检测方案(多肽合成仪)》用到的仪器有CEM LibertyBlue 2.0 全自动微波多肽合成仪。
我要纠错
相关方案





 咨询
咨询