方案详情文
智能文字提取功能测试中
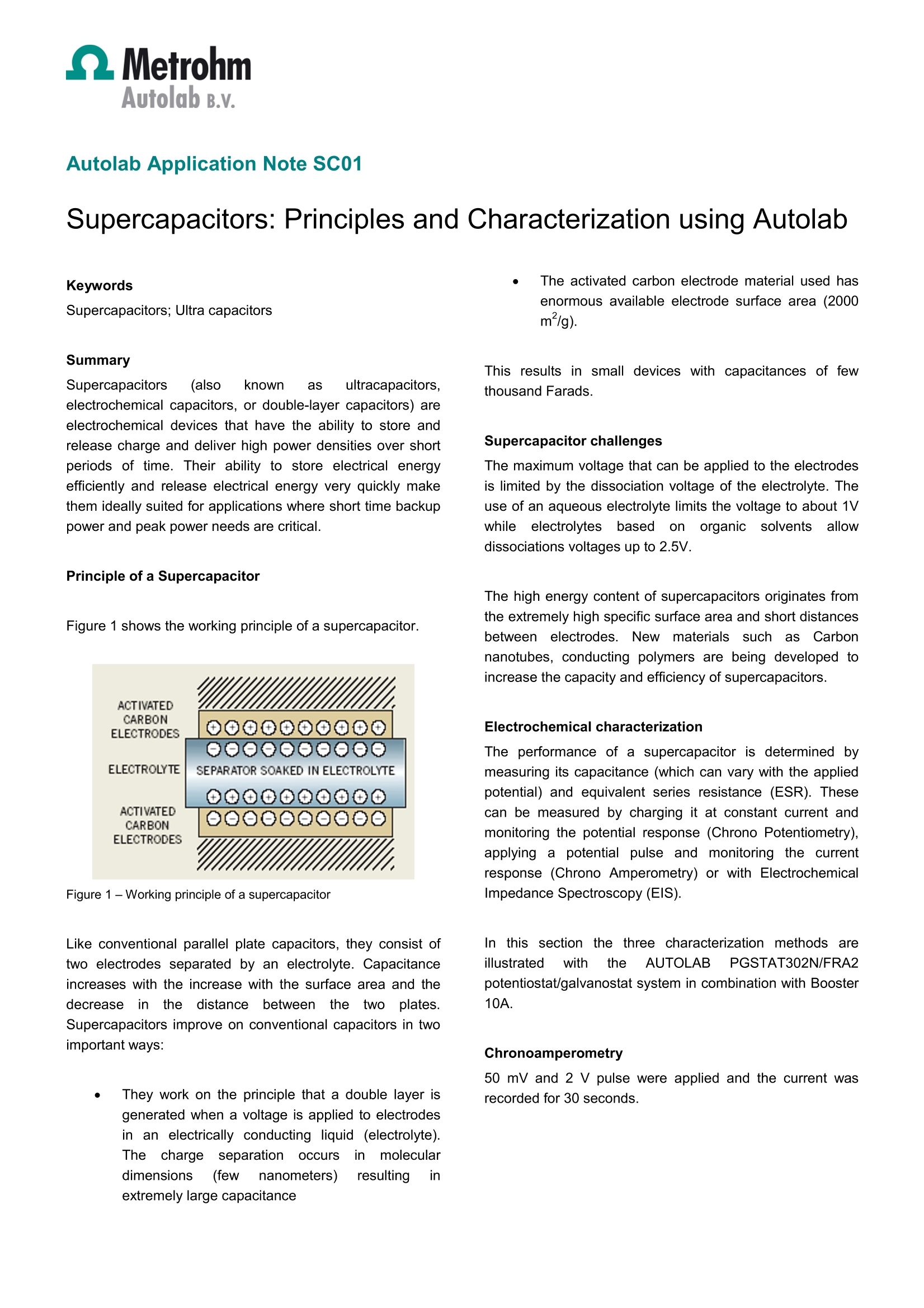
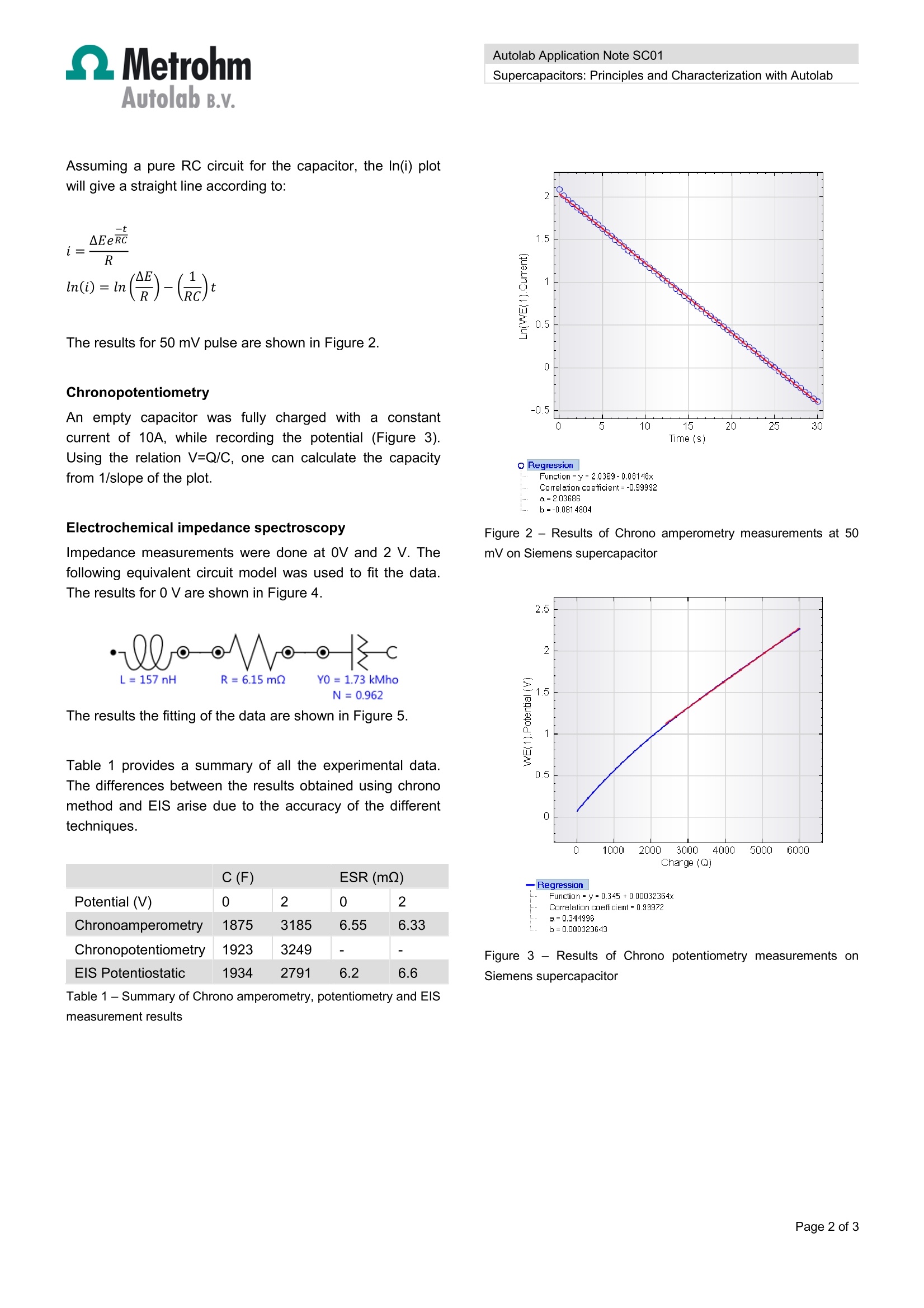
Autolab Application Note SC01Autolab B.v. Autolab Application Note SC01Supercapacitors: Principles and Characterization with Autolab Autolab Application Note SC01 Supercapacitors: Principles and Characterization using Autolab Summary Supercapacitors ((ealso known as ultracapacitors,electrochemical capacitors, or double-layer capacitors) areelectrochemical devices that have the ability to store andrelease charge and deliver high power densities over shortperiods of time. Their ability to store electrical energyefficiently and release electrical energy very quickly makethem ideally suited for applications where short time backuppower and peak power needs are critical. Principle of a Supercapacitor Figure 1 shows the working principle of a supercapacitor. Figure 1- Working principle of a supercapacitor Like conventional parallel plate capacitors, they consist oftwo electrodes separated by an electrolyte. Capacitanceincreases with the increase with the surface area and thedecreaseintthedistancebetween theetwoplates.Supercapacitors improve on conventional capacitors in twoimportant ways: ● They work on the principle that a double layer isgenerated when a voltage is applied to electrodesiin an electrically conducting liquid (electrolyte).The chargeseparationoccursirinmoleculardimensions (few nanometers) resulting inextremely large capacitance ● The activated carbon electrode material used hasenormous available electrode surface area (2000m²/g). This results in small devices with capacitances of fewthousand Farads. Supercapacitor challenges The maximum voltage that can be applied to the electrodesis limited by the dissociation voltage of the electrolyte. Theuse of an aqueous electrolyte limits the voltage to about 1Vwhile electrolytes basedon organicsolventsaallowdissociations voltages up to 2.5V. The high energy content of supercapacitors originates fromthe extremely high specific surface area and short distancesbetween electrodes. NNewmaterialsls such as Carbonnanotubes, conducting polymers are being developed toincrease the capacity and efficiency of supercapacitors. Electrochemical characterization The performance of a supercapacitor is determined bymeasuring its capacitance (which can vary with the appliedpotential) and equivalent series resistance (ESR). Thesecan be measured by charging it at constant current andmonitoring the potential response (Chrono Potentiometry),applying a potential pulse and monitoring the currentresponse (Chrono Amperometry) or with ElectrochemicalImpedance Spectroscopy (EIS). In this section the three characterization methods areillustrated with the AUTOLAB PGSTAT302N/FRA2potentiostat/galvanostat system in combination with Booster10A. Chronoamperometry 50 mV and 2 V pulse were applied and the current wasrecorded for 30 seconds. Assuming a pure RC circuit for the capacitor, the In(i) plotwill give a straight line according to: AEeR元Ci=Rln(i)=ln()-(c) The results for 50 mV pulse are shown in Figure 2. Chronopotentiometry An empty capacitor was fully charged with a constantcurrent of 10A, while recording the potential (Figure 3).Using the relation V=Q/C, one can calculate the capacityfrom 1/slope of the plot. Electrochemical impedance spectroscopy Impedance measurements were done at 0V and 2 V. Thefollowing equivalent circuit model was used to fit the data.The results for 0 V are shown in Figure 4. The results the fitting of the data are shown in Figure 5. Table 1 provides a summary of all the experimental data.The differences between the results obtained using chronomethod and EIS arise due to the accuracy of the differenttechniques. C(F) ESR(mQ) Potential (V) 0 2 2 Chronoamperometry 1875 3185 6.55 6.33 Chronopotentiometry 1923 3249 - - EIS Potentiostatic 1934 2791 6.2 6.6 Table 1-Summary of Chrono amperometry, potentiometry and ElSmeasurement results Q Regression Function=y=2.0369-0.08148xCorrelation coefficient=-0.99992a=2.03686 b=-0.0814804 Figure 2 -Results of Chrono amperometry measurements at 50mV on Siemens supercapacitor Correlation coefficient=0.99972a=0.344996 b=0.000323643 Figure 3 - Results of Chrono potentiometry measurements onSiemens supercapacitor Figure 4 -Results of EIS measurements at 0 V on Siemenssupercapacitor X File Edit Element Parameter Value Estimated Error (%) L1 L 1.5659E-07 0.927 R1 0.006154 0.305 Q1 Y0 1733.7 1.826 N 0.96247 0.577 0.032959 Figure 5 -Summary of the fitted values obtained from theimpedance spectroscopy data Date 1 July 2011 Page of 超级电容器是一种电化学装置,能够储存和释放电荷并在短时间内提供高功率密度。它们能够高效地存储电能并快速释放电能,使其非常适合短时间备用电源和峰值功率需求至关重要的应用。本文详细解释了超级电容器的理论基础,并用电化学工作站对其进行表征。
关闭-
1/3
-
2/3
还剩1页未读,是否继续阅读?
继续免费阅读全文产品配置单
瑞士万通中国有限公司为您提供《超级电容器中电化学检测方案(电化学工作站)》,该方案主要用于电子元器件产品中电化学检测,参考标准《暂无》,《超级电容器中电化学检测方案(电化学工作站)》用到的仪器有瑞士万通PGSTAT302N电化学工作站。
我要纠错
推荐专场
相关方案





 咨询
咨询

