方案详情文
智能文字提取功能测试中
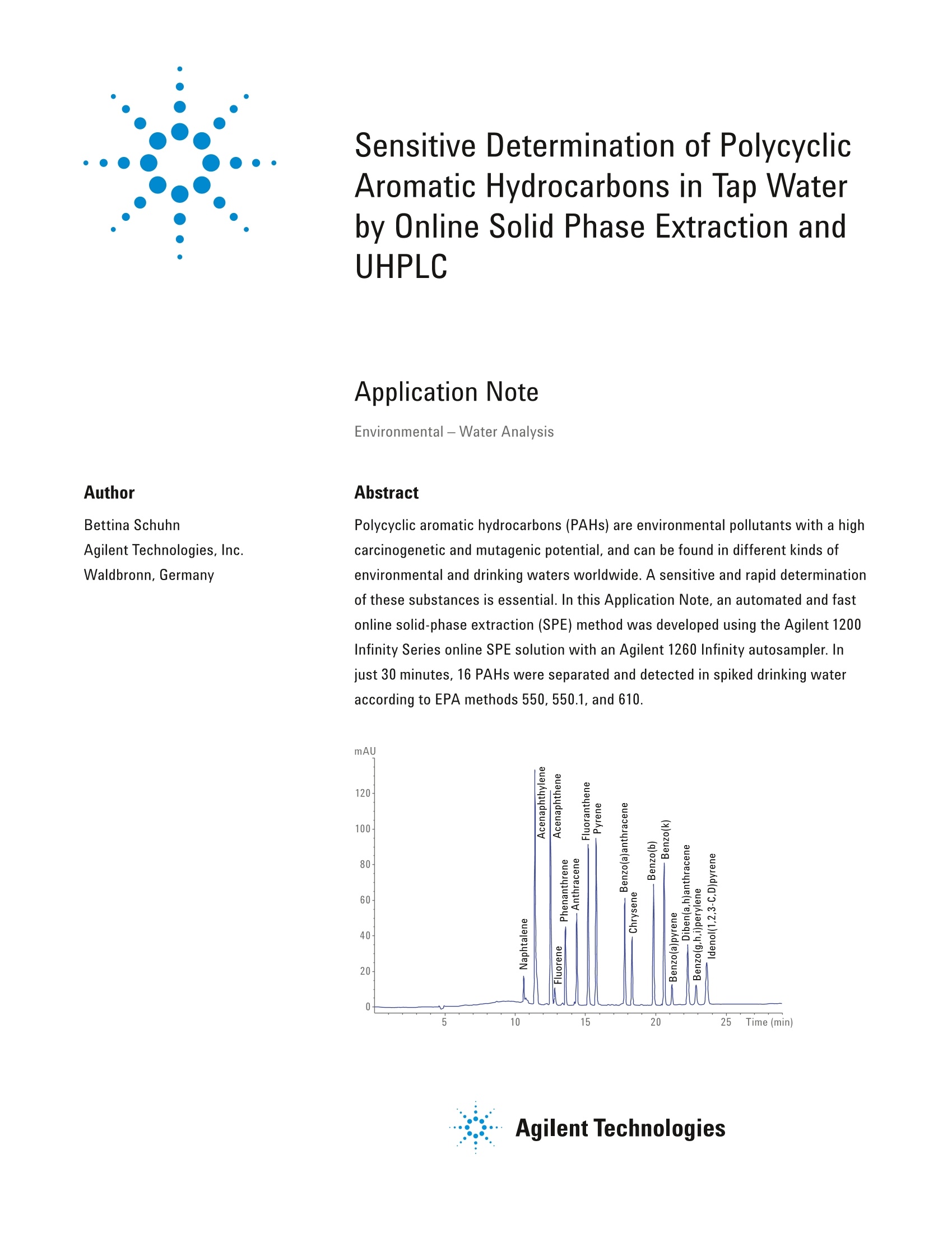
Sensitive Determination of PolycyclicAromatic Hydrocarbons in Tap Waterby Online Solid Phase Extraction andUHPLC Application Note Environmental-Water Analysis Author Abstract Bettina SchuhnAgilent Technologies, Inc. Waldbronn, Germany Polycyclic aromatic hydrocarbons (PAHs) are environmental pollutants with a highcarcinogenetic and mutagenic potential, and can be found in different kinds ofenvironmental and drinking waters worldwide. A sensitive and rapid determinationof these substances is essential. In this Application Note, an automated and fastonline solid-phase extraction (SPE) method was developed using the Agilent 1200Infinity Series online SPE solution with an Agilent 1260 Infinity autosampler. Injust 30 minutes, 16 PAHs were separated and detected in spiked drinking wateraccording to EPA methods 550, 550.1, and 610. mAU Agilent Technologies Introduction Polycyclic aromatic hydrocarbons (PAHs)are ubiquitous in the environment andformed by the incomplete burning andcombustion of hydrocarbons such aswoods, gases, fuel for automobiles, andcigarettes. They are usually found asa mixture by adsorption onto airborneparticles. These particles are part of raindrops and snow, and can be found insediment, surface water, and drinkingwater. Another major source of PAHs indrinking water is leaching from liningsof water storage tanks and distributionlines. Seven PAHs are classified asprobable human carcinogens by theEnvironmental Protection Agency(US EPA):benz[a]anthracene,benzo[a]pyrene,benzo[b]fluoranthene,benzo[k]fluoranthene, chrysene,dibenz[a,h]anthracene, andindeno[1,2,3-cd]pyrene.Benzo[a]pyreneis known as one of the most dangerouscarcinogens, and is used as an indicatorfor the presence of PAH. The EPA, forexample, uses mathematical modelsto estimate the probability of a persondeveloping cancer from ingesting watercontaining a specified concentration ofa chemical. For benzo[a]pyrene, the EPAhas calculated an oral cancer slope factorof 7.3 (mg/kg/d)2. Consequently, the determination of PAHsin environmental and drinking wateris highly important. According to EPA(method 550, 550.1 and 610) and theEuropean Union legislation, 16 parentPAHs should be monitored3.4.5,6 Thesemethods are based on offline solid-phaseextraction (SPE) or liquid-liquid extraction(LLE), and have run times greater than60 minutes. Both offline SPE and LLErequire high sample volumes (1 L), andare labor intensive and time-consuming.Additionally, high amounts of organicsolvents are needed, which may resultin human health effects, and are ofenvironmental concern. An effective alternative is online SPE dueto cost, time, and solvent savings. Thesample preparation and disposable wastecan be reduced to a minimum, and watersamples of less than 2 mL are sufficientfor the highly sensitive determination ofPAHs in drinking water. The overall runtime in this work, which included theenrichment process and analytical run,was just 30 minutes. This Application Note shows anddescribes the sensitive, fast, andreproducible online SPE analysis of16 PAHs spiked in drinking water. Goodrecovery rates, linearity, and precisionfor retention time and area are shown.The UHPLC method included both diodearray and fluorescence detection modes,and was developed on an Agilent 1200Infinity Series online SPE system, basedon an Agilent 1260 Infinity LC with anAgilent 1260 Infinity autosampler for moresample capacity (2×18,6-mL vials). Experimental Instrumentation An Agilent 1200 Infinity Series Online SPEsystem comprising the following moduleswas used for the experiments. Agilent 1260 Infinity autosamplerwith sample cooler (G7129A withoption#100) Agilent 1260 Infinity binary pumpwith degasser (G1312B andG1322A) Agilent 1260 Infinity quaternarypump (G1311B) Agilent 1290 Infinity thermostattedcolumn compartment (G1316B) Agilent 1290 Infinity Flexible Cube(G4227A) Agilent 1260 Infinity diode arraydetector (G4212B) Agilent 1290 Infinity fluorescencedetector (G1321B) Software Agilent OpenLab CDS ChemStationEdition for LC and LC/MS systems,Rev. C.01.07 [22] Instrumentation setup The online SPE Solution is based on the1290 Infinity Flexible Cube that housesre-usable SPE cartridges and up to twovalves. The two valves enable the choiceof switching between direct injection andonline SPE methods without replumbingthe system. The conventional setup usesthe built-in piston pump for loading andcleaning the cartridges. The integratedsingle-piston pump has a maximumpressure of 60 bar. As two analyticalcolumns were used as enrichmentcolumns and, therefore, the backpressurewas too high, the system was modified bythe addition of a 1260 Infinity quaternarypump for loading and cleaning theenrichment column. The built-in pumpof the 1290 Infinity Flexible Cube wasbypassed (Figure 1). Another addition to the system is the1260 Infinity autosampler instead of the1260 Infinity standard autosampler thatwas previously used. The 1260 Infinityautosampler shortens injection cyclesto 18 seconds, lowers carryover withan automated needle wash station, andenlarges sample capacity to 36× 6-mLvials or 132 ×2-mL vials. The 1260 Infinityautosampler can be equipped with anintegrated cooler. With a 900-pL loop,analytical head, and a multidraw seatcapillary of 1,500 pL (G1313-98308), the1260 Infinity autosampler can inject up to1,800 pL. The setup with two valves installedenables direct injection and online SPEmethods without replumbing the system.For direct injection methods, the leftvalve has to be in position 1 to 2 todirectly connect the binary pump to theautosampler. In this mode,the right valveis disabled, and no analytical flow passesthe enrichment columns. Otherwise, position 1 to 10 on the leftvalve enables the online SPE enrichmentprocess and subsequent chromatographicseparation on the analytical column anddetection of compounds. Figure 1 showsthe schematic flow chart of this setup.The sample is alternatively loaded toone of the enrichment columns by thequaternary pump, while the binary pumpequilibrates the analytical column. After switching the right valve, the loadedenrichment column is connected to thebinary pump, and the compounds willbe eluted by the gradient and detected by the UV and fluorescence detectors.At the same time, the quaternary pumpcleans and re-equilibrates the secondenrichment column for the next run. Figure 1. Schematic of online SPE enrichment process. Loading and cleaning is done by the Agilent 1260Infinity quaternary pump while the Agilent 1260 Infinity binary pump delivers the elution gradient. Chromatographic conditions Parameter Value Analytical column Agilent ZORBAX Eclipse PAH 4.6 ×150 mm, 3.5 pm (p/n 959961-918) Column temperature 25°C Gradient A) Water, B) Acetonitrile 0 minutes,40 %B at 1.3 mL/min 5 minutes, 40 %B 20 minutes, 100 %B 30 minutes, 100 %B 30.1 minutes, 40 %B Posttime 6 minutes Injection volume 1,800 pL(online SPE), needle wash 3 seconds with acetonitrile Autosampler temperature 18°C Online SPE enrichment Polaris 5 C18-A, 3.0×50 mm (p/n A2000050X030) column Online SPE enrichment A) Water, B) Acetonitrile method 0 minutes,20 %B at 1.3 mL/min 3 minutes, 20%B 4 minutes at 0 mL/min 5 minutes,100 %B at 1 mL/min 9 minutes, 100 %B 9.1 minutes, 20 %B at 1 mL/min 15 minutes, 20 %B 15.1 minutes, 20 %B at 0 mL/min Diode array detector 230 nm, bandwidth 4 nm, reference 400 nm, reference band width 100 nm, 10 Hz Fluorescence detector Multisignal acquisition, set at A =260 nm and Am=350 nm (FLD A), 330 nm (FLD B), 440 nm (FLD C), 500 nm (FLD D), 18.51 Hz, PMT 13 Chemicals All solvents used were LC grade.Acetonitrile was purchased from Merck,Germany. Fresh ultrapure water wasobtained from a Milli-Q Integral systemequipped with an LC-Pak Polisher and a0.22-pm membrane point-of-use cartridge(Millipak). Sample A PAH mixture of 16 compoundsaccording to EPA 550 (p/n 8500-6035)was purchased from Agilent Technologies.Drinking water was from Waldbronn,Germany, and was spiked with PAHsin different concentrations. For thecalibration in drinking water, sevenstandards were prepared by diluting astock solution of 500 pg/mL to 10, 5, 1,0.5,0.1, 0.05, and 0.01 ug/L. Results and Discussion A suite of 16 PAHs was spiked atdifferent levels in drinking water.Fifteen of these PAHs can be detectedby fluorescence, which offers a bettersensitivity than UV. Acenaphthylene hasno fluorescence activity, and requires UVdetection. At first, the chromatographicseparation was optimized with directinjection methods and UV detection, thenlater on transferred to online SPE andUV/fluorescence detection. For the determination of method precisionfor all PAHs and online SPE, sevenconsecutive runs with 1 pg/L standardwere injected. Table 1 shows theretention time (RT) detection conditionsas well as RT and area precision for allPAHs. RT reproducibility (RSD) was less than 0.09 %, and area reproducibilityless than 6.0 %, and, thus, shows goodprecision for the online SPE method.Figure 2 shows a chromatogram with UVand different FLD wavelengths for thedetection of 16 PAHs in drinking water. Elution Retention RT RSD (%) Area RSD order Compound time (min) Detection (nm) (n=7) (%)(n=7) 1 Naphthalene 10.6 UV 230/Ref 400, 0.028 3.8 Ex 260, Em 330 2 Acenaphthylene 11.4 UV 230/Ref 400 0.025 0.9 3 Acenaphtene 12.5 Ex 260/Em 330 0.029 1.0 4 Fluorene 12.8 Ex 260/Em 330 0.031 0.5 5 Phenantrene 13.5 Ex 260/Em 350 0.035 0.9 6 Anthracene 14.3 Ex 260/Em 440 0.040 0.6 7 Fluoranthene 15.1 Ex260/Em 440 0.050 0.3 8 Pyrene 15.7 Ex 260/Em 440 0.050 6.0 9 Benzo(a)anthracene 17.7 Ex 260/Em 440 0.070 2.3 10 Chrysene 18.2 Ex 260/Em 350 0.071 0.8 11 Benzo(b)fluoranthene 19.7 Ex 260/Em 440 0.080 1.3 12 Benzo(k)fluoranthene 20.5 Ex 260/Em 440 0.090 1.1 13 Benzo(a)pyrene 22.1 Ex 260/Em 440 0.080 2.5 14 Dibenzo(a,h)anthracene 22.2 Ex 260/Em 440 0.011 2.0 15 Benzo(g,h,i)perylene 22.8 Ex 260/Em 440 0.013 1.4 16 Indeno(1,2,3-cd)pyrene 23.5 Ex 260/Em 500 0.015 3.9 mAU1 Calibration linearity was investigatedwith replicates for each concentrationlevel.In Table 2, different linearity rangesare shown for all PAHs. Linearity wasgood across the ranges, giving values of0.999 or better. Limits of detection (LODs)and limits of quantification (L0Qs) wereevaluated from the concentration of PAHsrequired to give at least a signal-to-noiseratio (S/N) of 3 and 10, respectively. LODs were between 0.2 and 23 ng/L.LOOs for all compounds werebetween 1 and 38 ng/L (except foracenaphthylene, with 77 ng/L because oflower sensitivity of UV detection). Figure 3 clearly shows the calibrationcurves of four PAHs (anthracene,benzo(a)anthracene, benzo(a)pyrene,andbenzo(b)fl uoranthene). The EPA has set a maximum contaminantlevel (MCL) for benzo(a)pyrene in drinkingwater at 0.2 pg/L. This MCL can beeasily achieved. The online SPE systemis 100 times more sensitive with aninjection volume of 1,800 pL. Elution Linearity LOD (S/N=3) LOQ(S/N=10) order Compound range(ug/L) R2 (pg/L) (ug/L) 1 Naphthalene 0.01-10 0.99834 0.0009 0.0031 2 Acenaphthylene 0.5-10 0.99992 0.0233 0.0775 3 Acenaphtene 0.01-5 0.99970 0.0018 0.0061 4 Fluorene 0.01-1 0.99932 0.0003 0.0009 5 Phenantrene 0.01-1 0.99932 0.0004 0.0013 6 Anthracene 0.01-1 0.99982 0.0009 0.0032 7 Fluoranthene 0.01-10 0.99995 0.0018 0.0058 8 Pyrene 0.05-10 0.99991 0.0028 0.0092 9 Benzo(a)anthracene 0.05-5 0.99991 0.0008 0.0028 10 Chrysene 0.01-10 0.99915 0.0039 0.0129 11 Benzo(b)fluoranthene 0.01-1 0.99996 0.0016 0.0053 12 Benzo(k)fluoranthene 0.01-1 0.99985 0.0008 0.0025 13 Benzo(a)pyrene 0.01-0.5 0.99985 0.0022 0.0074 14 Dibenzo(a,h)anthracene 0.01-10 0.99874 0.0019 0.0062 15 Benzo(g,h,i)perylene 0.05-10 0.99835 0.0109 0.0362 16 Indeno(1,2,3-cd)pyrene 0.05-10 0.99989 0.0114 0.0380 Figure 3. Calibration curves of anthracene, benzo(a)anthracene, benzo(a)pyrene, and benzo(b)fluoranthene in concentration ranges between 0.01 to 10 pg/L forthe online SPE method and an injection volume of 1,800 pL. To determine the recovery of the trappingprocess on the SPE cartridges, the samevolume (5pL) of a 250 ug/L solutionwas injected directly onto the analyticalcolumn and afterwards onto the SPEtrapping columns. Seven replicatesof each method were averaged andcompared. The recovery range wasbetween 90 and 110 % (Figure 4), withthe exception of benzo(a)pyrene andindeno(1,2,3-cd)pyrene, where therecovery was 140 and 73 %, respectively.This demonstrates that the online SPEmethod is perfectly suited for the analysisof PAHs in drinking water, because noanalyte is flushed out and lost during theenrichment process. The analysis of PAHs in water can befully automated with online solid-phaseextraction with a combination of UVand fluorescence detection. No samplepretreatment is necessary, and the overallrun time of 16 PAHs is just 30 minutes.Excellent recoveries were determinedfor all analytes. Detection limits werebetween 0.2 and 23 ng/L, with an1,800 pL injection, and quantificationlimits were in the range of 1 to 38 ng/L.Reproducibility for two alternatingenrichment columns was less than 6 %RSD with excellent correlation (typicallyR2=0.999), which demonstrates thatonline SPE is a perfect alternative totime-consuming and expensive offlineSPE. Figure 4. Recovery of the online SPE method in comparison to the direct injection method. References 1. Yoshiyuki Watabe et al., Trace leveldetermination of polycyclic aromatichydrocarbons in river water withautomated pretreatment HPLC,J. Sep.Sci. 2013,36,1128-1134 2. http://www.epa.gov/ttnatw01/hlthef/polycycl.html (accessedSeptember 1, 2015) US EPA (1990) Method 550,Determination of polycyclic aromatichydrocarbons in drinking water byliquid-liquid extraction and HPLC withcoupled ultraviolet and fluorescencedetection, publication numberPB91-146027 US EPA (1990) Method 550.1,Determination of polycyclic aromatichydrocarbons in drinking water byliquid-solid extraction and HPLC withcoupled ultraviolet and fluorescencedetection, publication numberPB91-146027 US EPA (1982) Method 610,Polynuclear Aromatic Hydrocarbons Lerda, D., Polycyclic aromatic hydrocarbons (PAHs) factsheet. European Commission, Joint Research Centre, Institute for Reference Materials and Measurements, Belgium, 2012. This information is subject to change without notice. @ Agilent Technologies, Inc.,2015 Published in the USA, October 1, 2015 5991-6288EN Agilent Technologies 多环芳烃(PAHs)是高污染物的环境污染物致癌和诱变的潜力,存在于不同种类的全球的环境和饮用水。 灵敏而快速的检测这些物质是必不可少的。 在本应用中,使用Agilent 1200Infinity系列在线SPE解决方案,结合Agilent 1260 Infinity自动进样器,开发了在线固相萃取(SPE)方法。 仅30分钟,就分离了16种PAH,并在加标的饮用水中检测到。
关闭-
1/8
-
2/8

还剩6页未读,是否继续阅读?
继续免费阅读全文产品配置单
安捷伦科技(中国)有限公司为您提供《自来水中多环芳烃检测方案(液相色谱仪)》,该方案主要用于环境水(除海水)中有机污染物检测,参考标准《暂无》,《自来水中多环芳烃检测方案(液相色谱仪)》用到的仪器有Agilent 1260 Infinity II 液相色谱系统。
我要纠错
相关方案



 咨询
咨询


