方案详情文
智能文字提取功能测试中
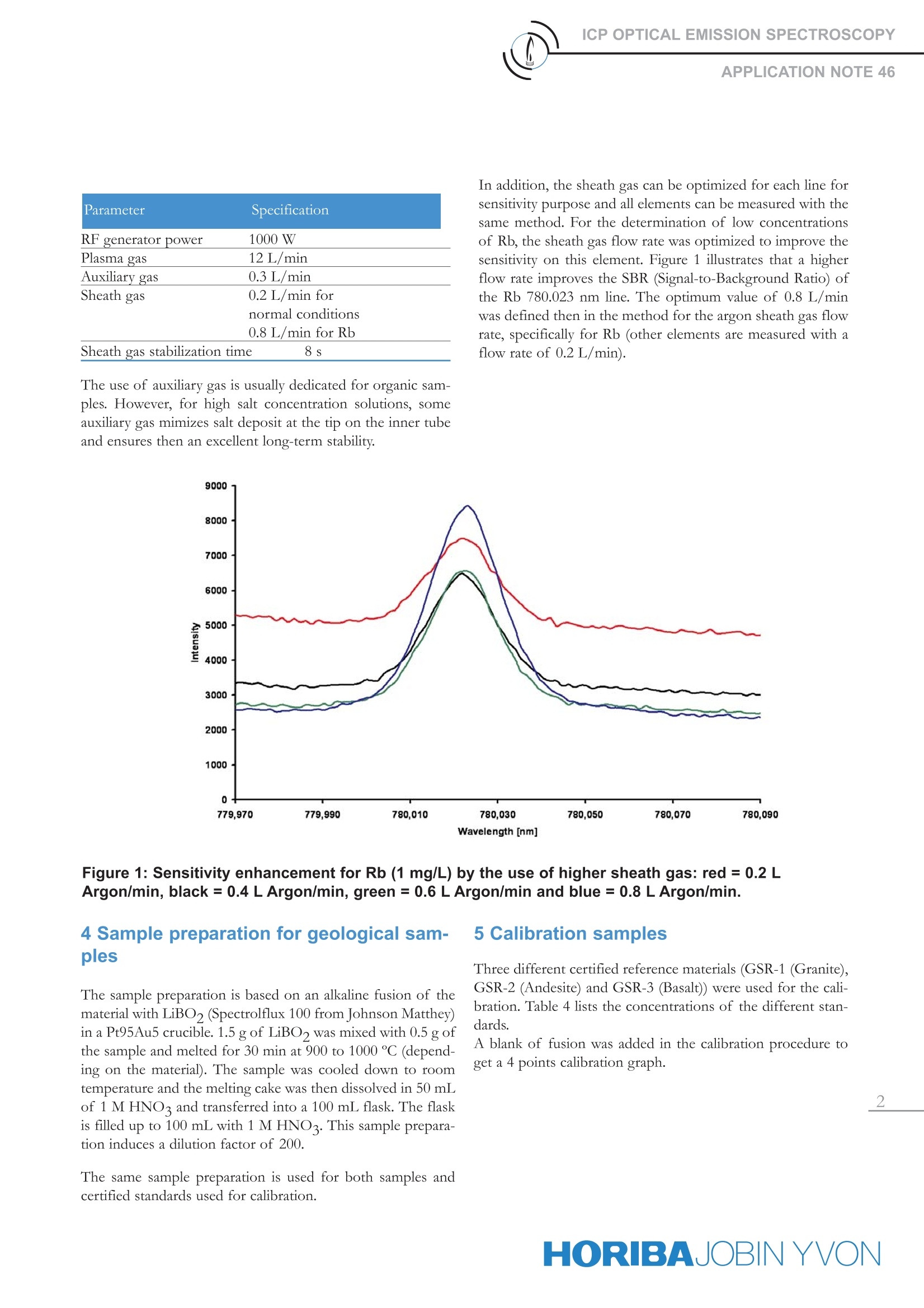
ICP ATOMIC EMISSION SPECTROSCOPYAPPLICATION NOTE 46 GEOLOGICAL SAMPLES BYACTIVA-M ICP-AES Prof. Richard Tessadri, Institute of Mineralogy and Petrography, University of Innsbruck, AustriaDr. Rainer Nehm, ICP Applications Specialist, HORIBA Jobin Yvon, Munich, GermanyAgnes Cosnier, ICP Product Manager, HORIBA Jobin Yvon, Longjumeau, France Keywords: geology, determination of Rb 1 Introduction This application note describes the analysis of several ele-ments (Ba, Be, Co, Cr, Cu, Nb,Ni, Rb, Sc, Sr,V, Y, Zn andZr) in geological samples, with the ACTIVA-M ICP-AESinstrument. Analysis of geological samples requires anICP-AES instrument with good resolution and robustness,to compensate for the variability of matrix and the influ-ence of the major elements. The material was dissolved by fusion with LiBO2 in aPt95Au5 crucible. The calibration was performed with 3different certified reference materials (GSR-1 (Granite),GSR-2 (Andesite) and GSR-3 (Basalt)) from IGGE, China.A Kimberlite sample (SARM 39) was then analyzed to val-idation the analytical methodology. For the determinationof low concentrations of Rb,a specific optimization wasrealized and is detailed. 2 Principle The elemental analysis of these samples was done byInductively Coupled PlasmaAtomicEmissionSpectrometry (ICP-AES). The sample, in liquid form, isnebulized and then transferred with an argon carrier gasstream to an argon plasma. The sample is dried, atomizedand ionized, whereby the atoms and ions are excited. Theintensity of the light is measured when the atoms or ionsreturn to lower levels of energy. Each element emits lightat characteristic wavelengths which can be used for theidentification of the element. The intensity of the light isused for the quantification. 3 Instrumental specifications and oper-ating conditions The work was done on the ACTIVA-M ICP-AES spec-trometer. The specifications of the instrument andsample introduction system are listed in Table 1and Table 2 respectively. For the determination ofelements in geological samples, which are dissolvedby alkaline fusion with LiBO2, it is strongly recom-mended to use a nebulizer suitable to high salt concentra- tion, to provide freedom from clogging. We used the OpalMist nebulizer, which is made of PFA and resistantHF solutions. The operating conditions of the instrumentare listed in Table 3. Table 1: Specifications of the ACTIVA-M ICP spec-trometer Parameter Specification Optical mounting Czerny Turner Focal length 0.64 m Spectral range 120 -800 nm Nitrogen purge Yes Resolution <10 pm up to 430 nm <18 pm in 430-800 nm Gratings Grating 1: 4343 grooves per mm Grating 2: 2400 Order 1st order Type of generator Solid state Frequency 40.68 MHz Generator cooling Air Observation Radial (view of total NAZ*) Control of gas flow rates By computer Control of pump speed By computer * Normal Analytical Zone Table 2: Specifications of the sample introductionsystem Parameter Specification Nebulizer OpalMist (PFA concentric) Nebulizer pressure 3 bar Nebulizer Argon flow 0.84 L/min Spray chamber Cyclonic (glass) Sample uptake 2 mL/min Argon humidifier No Injector tube diameter 3 mm Parameter Specification RF generator power 1000 W Plasma gas 12 L/min Auxiliary gas 0.3 L/min Sheath gas 0.2 L/min for normal conditions 0.8 L/min for Rb Sheath gas stabilization time 8s The use of auxiliary gas is usually dedicated for organic sam-ples. However, for high salt concentration solutions, someauxiliary gas mimizes salt deposit at the tip on the inner tubeand ensures then an excellent long-term stability. In addition, the sheath gas can be optimized for each line forsensitivity purpose and all elements can be measured with thesame method. For the determination of low concentrationsof Rb, the sheath gas flow rate was optimized to improve thesensitivity on this element. Figure 1 illustrates that a higherflow rate improves the SBR (Signal-to-Background Ratio) ofthe Rb 780.023 nm line. The optimum value of 0.8 L/minwas defined then in the method for the argon sheath gas flowrate, specifically for Rb (other elements are measured with aflow rate of 0.2L/min). Figure 1: Sensitivity enhancement for Rb (1 mg/L) by the use of higher sheath gas: red= 0.2 LArgon/min, black = 0.4 L Argon/min, green = 0.6 L Argon/min and blue=0.8 L Argon/min. 4 Sample preparation for geological sam-ples The sample preparation is based on an alkaline fusion of thematerial with LiBO, (Spectrolflux 100 from Johnson Matthey)in a Pt95Au5 crucible. 1.5 g of LiBO was mixed with 0.5 g ofthe sample and melted for 30 min at 900 to 1000 ℃ (depend-ing on the material). The sample was cooled down to roomtemperature and the melting cake was then dissolved in 50 mLof 1 M HNO3 and transferred into a 100 mL flask. The flaskis filled up to 100 mL with 1 M HNO3. This sample prepara-tion induces a dilution factor of 200. The same sample preparation is used for both samples andcertified standards used for calibration. Three different certified reference materials (GSR-1 (Granite),GSR-2 (Andesite) and GSR-3 (Basalt)) were used for the cali-bration. Table 4 lists the concentrations of the different stan-dards. A blank of fusion was added in the calibration procedure toget a 4 points calibration graph. Table 4: Concentrations of the different standards (val-ues in the solid) Element Wavlength GSR-1 GSR-2 GSR-3 nm Granite Andesite Basalt ppm ppm ppm Ba 455.403 343 1020 526 Be 313.042 12.4 1.1 2.5 Co 228.616 3.4 13.2 46.5 Cr 267.716 5.0 32.4 134 Cu 324.754 3.2 55.4 48.6 Nb 316.340 40.0 6.8 68.0 Ni 231.604 2.3 17.0 140 Rb 780.023 466 37.6 37.0 Sc 361.384 6.1 9.5 15.2 Sr 421.552 106 790 1100 292.402 24.0 95.5 167 Y 371.029 62.0 9.3 22.0 Zn 213.856 28.0 71.0 150 Zr 343.823 167 99.0 277 6 Results for the geological samples The results of a Kimberlite sample (SARM 39) are listed inTable 5. The good recoveries obtained, even for low concen-trations, validate the global analytical methodology (samplepreparation + analytical method). Table 5: Concentrations of the SARM 39 certified sam-ple (values in the solid) Element SARM 39 SARM Recovery Kimberlite Kimberlite measured certified % ppm ppm Ba 1740 1700 97.7 Be 1 1 100 Co 75 77 102.6 Cr 1264 1300 102.8 Cu 55 58 105.4 Nb 108 110 101.9 Ni 1021 994 97.4 Rb 51 52 102.0 Sc 14 13 92.9 Sr 1369 1400 102.3 111 109 98.2 Y 16 17 106.3 Zn 67 70 104.5 Zr 231 239 103.5 7 Conclusion This application note demonstrates the performance of the ACTIVA-M ICP instrument for geological samples.In addition, the ACTIVA-M instrument can offer a completeassistance for the development and validation of the method,based on the multi-line analysis concept which is described inReference [1]. [1]Application of Unique Software Tools Dedicated to MultilineAnalysis by CCD-Based ICP-AES for Geological Samples, AgnesCosnier, Jean-Micbel Mermet, Sebastien Velasguey and SopbieLebouil; Applications of ICPICP-MS Tecbnigues for TodaysSpectroscopist, October 2007 HORIBAJOBIN YVON ORIBAJOBIN YVON
关闭-
1/3

-
2/3
还剩1页未读,是否继续阅读?
继续免费阅读全文产品配置单
HORIBA(中国)为您提供《土壤中(类)金属及其化合物检测方案 》,该方案主要用于土壤中(类)金属及其化合物检测,参考标准《暂无》,《土壤中(类)金属及其化合物检测方案 》用到的仪器有HORIBA Ultima Expert高性能ICP光谱仪。
我要纠错
推荐专场
相关方案






 咨询
咨询




