方案详情文
智能文字提取功能测试中
STANDARD OPERATING PROCEDURESPAGE: 1 of 37REV: 2.0DATE:01/23/06 STANDARD OPERATING PROCEDURESDATE:01/23/06 SOP: 1801 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENTSAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) CONTENTS 1.0 SCOPE AND APPLICATION* 2.0 METHOD SUMMARY 2.1 Water Samples 2.2 Soil Samples 3.0 SAMPLE PRESERVATION, CONTAINERS,HANDLING, AND STORAGE 3.l Sample Storage 3.2 Holding Time 4.0 INTERFERENCES AND POTENTIAL PROBLEMS 5.0 EQUIPMENT/APPARATUS 6.0 REAGENTS 7.0 PROCEDURES Sample Preparation and Extraction for Water Sample Preparation and Extraction for Soil Sample Concentration for Water and Soil Extracts 7.4 Total Solids 7.5 Gel Permeation Chromatography Cleanup 7.6 Florisil Cleanup 7.7 Tetrabutylammonium (TBA)-Sulfite Cleanup 7.8 Copper Cleanup 7.9 Sulfuric Acid Cleanup 7.10 GC/ECD Conditions 7.11 Retention Time Windows 7.12 Standard and Sample Analysis* SOP: 1801 PAGE: 2 of 37 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENTSAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) CONTENTS (cont) 7.13 Evaluation of Chromatograms 7.13.1 Standard/Sample Chromatograms 7.13.2 PCB Identification 7.14 Sample Dilution 8.0 CALCULATIONS 8.1 Reporting Limit for Water 8.2 Reporting Limit for Soil Sample Concentration for Water Using Internal Standard Method Sample Concentration for Water Using External Standard Method 8.5 Sample Concentration for Soil Using Internal Standard Method 8.6 Sample Concentration for Soil Using External Standard Method 8.7 Surrogate Spike Recoveries 8.8 Matrix Spike Recoveries 8.9 Laboratory Control Sample Recoveries 8.10 Dixon's Criterion 9.0 QUALITY ASSURANCE/QUALITY CONTROL Holding Time Identification of Target Compounds Initial Calibration for Target Compounds and Surrogates* 9.4 Continuing Calibration for Target Compounds and Surrogates 9.5 Retention Time Windows 9.6 Analytical Sequence 9.7 Method Blank and Laboratory Control Sample* 9.8 Surrogate Recoveries 9.9 Internal Standards 9.10 Matrix Spike and Matrix Spike Duplicate Analysis* 9.11 Initial Demonstration of Capability 9.12 Dilution Analysis 9.13 Reporting Limit 9.14 Method Detection Limit Studies 9.15 Nonconformance Memo SOP: 1801 PAGE: 3 of37 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) CONTENTS (cont’d) 10.0 DATA VALIDATION 11.0 HEALTH AND SAFETY 12.0 REFERENCES* 13.0 APPENDICES A-Tables* B-Figure C-Sothern Extractor Operating Conditions * These sections affected by Revision 2.0 SUPERSEDES: SOP #1801, Revision 1.0, 11/27/05: U.S. EPA Contract EP-W-09-031 SOP: 1801 PAGE: 4 of 37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 1.0 SCOPE AND APPLICATION This standard operating procedure (SOP) is applicable to the determination of polychlorinated biphenyls(PCBs) in water and soil/sediment matrices, using a gas chromatograph (GC) with a narrow-bore fused silicacolumn and an electron capture detector (ECD). This SOP is based on Environmental Protection Agency(EPA) Methods SW846/3510C/3540C/3541/8000B/8082 and those requirements set forth in the latestapproved version of the National Environmental Laboratory Accreditation Committee (NELAC) QualitySystems section. Extracts may be subjected to optional cleanup procedures (Florisil, gel permeationchromatography [GPC], tetrabutylammonium [TBA] sulfite, activated copper powder or acid) based onEPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A.The compounds of interest and typicalreporting limits (RLs) in water and soil/sediment matrices are found in Table 1, Appendix A. This method may not be changed without the expressed approval of the Organic Group Leader, theAnalytical Section Leader and the Quality Assurance Officer (QAO). Only those versions issued throughthe SERAS document control system may be used. Modifications made to the procedure due tointerferences in the samples or for any other reason must be documented in the case narrative and on anonconformance memo. 2.0 METHOD SUMMARY 2.1 Water Samples Approximately 1 liter (L) of a water sample is serially extracted at a neutral pH with methylenechloride. The extract is concentrated to 10 milliliters (mL), then 60 mL of hexane is added as anexchange solvent, and the extract is concentrated to a final volume of 1 mL. The extracts areanalyzed for PCBs using GC/ECD. A second column confirmation is optional for PCB analysis. 2.2 Soil Samples Approximately 30 grams (g) of a soil/sediment sample mixed with 30 g of anhydrous sodium sulfateis extracted with 140 milliliters (mL) of 1:1 acetone/hexane using a Soxtherm extractor for 2 hoursor 300 milliliters (mL) of 1:1 acetone/hexane using a Soxhlet extractor for 16 hours. The extract isconcentrated to 10 mL, 60 mL of hexane is added as an exchange solvent, and the extract isconcentrated to a final volume of5 mL. The extracts are analyzed for PCBs using GC/ECD.,lAsecond column confirmation is optional for PCB analysis. 3.0 SAMPLE PRESERVATION, CONTAINERS, HANDLING, AND STORAGE 3.1 Sample Storage Water samples should be collected in 1-L amber glass containers fitted with Teflon-lined caps.Soil samples should be collected in wide-mouth glass containers with Teflon-lined caps. From the time of collection until after analysis, extracts and unused samples must be protected fromlight and refrigerated at 4±2 degrees Celsius (C) for the periods specified by SERAS Task Leader(TL) and/or the Work Assignment Manager (WAM) for the project. SOP: 1801 PAGE: 5 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) Samples and sample extracts must be stored separately from standards in an atmosphere free of allpotential contaminants. 3.2 Holding Time Extraction of water and soil/sediment samples must be completed within 7 days from the date ofcollection and analysis completed within 40 days of sample extraction. 4.0 INTERFERENCES AND POTENTIAL PROBLEMS Solvents, reagents, glassware, and other sample processing hardware may yield artifacts and/or interferencesin the sample extracts for analysis.S.All of these materials must be demonstrated to be free from interferencesunder the conditions of the analysis by analyzing laboratory reagent blanks on a routine basis.S.Interferencesco-extracted from the samples may vary considerably from sample to sample. Cleanup procedures may benecessary if the extract contains analytes that interfere with quantitation or peak separation. Phthalate esters are present in many types of products commonly found in the laboratory. Some plastics, inparticular, must be avoided because phthalates are commonly used as plasticizers and are easily extractedfrom plastic materials.Serious phthalate contamination may result at any time if consistent quality controlis not practiced. Soap residue on glassware may cause degradation of certain analytes. This problem is especiallypronounced with glassware that may be difficult to rinse. These items should be hand-rinsed very carefullyto avoid this problem. Elemental sulfur is encountered in many sediment samples such as marine algae and some industrial wastes.Sulfur will be quite evident in gas chromatograms obtained from electron capture detectors.If the GC isoperated under normal conditions for PCBs analysis, the sulfur interference can completely mask the regionfrom the solvent peak through most ofthe Aroclor peaks. Three techniques, GPC cleanup, activated copperpowder, or TBA sulfite for the elimination of sulfur may be used. Florisil cleanup may be used to reducematrix interferences caused by polar compounds. Weathering ofPCBs in the environment may alter the pattern of the PCBs to the point where the pattern is notrecognizable..“Weathering”is defined as a change in the typical PCB pattern..Selected Aroclor peaks maybe used for quantitation when a sample exhibits a pattern similar to this effect. In some instances, the presence of multiple PCBs may affect the identification and quantitation of all PCBspresent in a sample. In this case, the analyst will note interferences/anomalies in the case narrative anddocument the reasoning for reporting the Aroclors present. 5.0 EQUIPMENT/APPARATUS The following equipment/apparatus is required: Soxtherm extractor, including its accessories (e.g., extraction flask, sample holding vessel, chiller, etc.), SOP: 1801 PAGE: 6 of37 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) manufactured by Gerhardt or equivalent Waters GPC instrument or equivalent Separatory funnel, 2000 mL with stopcock (glass or Teflon). Erlenmeyer flasks, 500 mL Graduated cylinder, 1-L, Class A Buchner funnels. Bench top shaker (Glas-Col) or equivalent. Soxhlet extractor, 40 millimeter (mm) inner diameter (ID), with 500-mL round bottom flask, fits 45/50condenser Teflon boiling chips, approximately 10/40 mesh, rinsed three times with methylene chloride Spoon and/or spatula, stainless steel or Teflon Glass container(s) Glass wool, Pyrex baked at 400℃ for 2 hours Balance, capable of accurately weighing 100 g to the nearest 0.01 g Kuderna-Danish (K-D) apparatus, consisting of a 10-mL graduated concentrator tube, 500-mLevaporation flask, and three-ball macro Snyder column Water bath heated with concentric ring cover, capable of maintaining temperature within ±2C. Thebath should be used in a hood. Disposable glass pasteur pipettes Nitrogen evaporation device, equipped with a water bath that can be maintained at 35-40°C (N-Evap byOrganomation Associations Model Number 111 or equivalent) ● TurboVap concentrator, with concentrator cells and racks Clean Bath solution, for use in TurboVap II concentrator Drying oven Desiccators SOP: 1801 PAGE: 7 of37 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) Vials and caps, 2 mL for GC autosampler Vials, 4-mL,for GPC cleanup ● Test tubes with screw caps, 25 mL °1pH paper, wide range. Ring stand Gas chromatograph-An analytical system complete with GC and all required accessories includingsyringes, autosampler, analytical columns, gases, an electron capture detector, and data system. A datasystem is required for measuring peak areas or peak heights and recording retention times. RTX-XLB fused silica capillary column, 30 meter(m) x 0.32 mm ID, 0.50 micron (um) filmThickness or equivalent RTX-CLPesticides fused silica capillary column, 30 mx 0.32 mm ID, 0.50 um film thickness orEquivalent Cellulose/Fiberglass thimbles, pre-washed with methylene chloride Teflon filters, 0.45um, for filtering extracts for gel permeation chromatography (GPC) cleanup (GelmanAcrodisc CR or equivalent) Florisil cartridge, 12-mL tube (Supelco CAT # 57155 or equivalent) Visiprap SPE Vacuum manifold, 12 port or equivalent Valve liners, disposable or equivalent. Syringes, miscellaneous Class “S" weight for balance calibration 6.0 REAGENTS Sodium Sulfate, anhydrous granular reagent grade, heated at 400 C for four hours, cooled in adesiccator, and stored in a glass bottle Methylene Chloride, pesticide residue analysis grade or equivalent Hexane, pesticide residue analysis grade or equivalent SOP: 1801 PAGE: 8 of 37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) Acetone, pesticide residue analysis grade or equivalent Methanol, pesticide residue analysis grade or equivalent 2-Propanol, pesticide residue analysis grade or equivalent Tetrabutylammonium sulfite solution - Prepare by dissolving 3.39 g of tetrabutylammoniumhydrogen sulfate in 100 mL ofreagent water. Extract this solution three times with 20 mL portionsof hexanes to remove any impurities. IDiscard the hexane layer and add 25g of sodium sulfite to theaqueous layer. Store this solution at room temperature. Copper powder, activated, commercially available Pesticide/PCB Internal Standard Solution. Prepare a solution containing4,4'-Dibromooctafluorobiphenyl, 4,4'- Dibromobiphenyl, and 3,3',4,4'-Tetrabromobiphenyl atconcentration of 5 microgram/milliliter (ug/mL) in hexane. PCB Stock Calibration Standards, 1000 pg/mL commerciallyavailable. PCB Working Calibration Standards - Prepare a minimum of five concentration levels at 0.25, 0.5,1.0, 2.0, and 5.0 milligrams/liter (mg/L). Each calibration standard must contain surrogates at aconcentration range of 20, 50, 100,200 and 500 micrograms/liter (ug/L) and contain thepesticide/PCB internal standard compounds at concentration of100 ug/L. Surrogate Stock Standard, 200 mg/L, commercially available. Pesticide/PCB Surrogate Working Solution. - Prepare a solution containing decachlorobiphenyl(DCBP) and 2,4,5,6-tetrachloro-meta-xylene (TCMX)at a concentration of 0.2 ug/mL (forwater)and 2 pg/mL (for soil) in methanol or acetoie. Stock PCB Matrix Spiking Solution, 1000 mg/L, commercially available, source must beindependent of the calibration standards. PCB Matrix Spike Working Solution - Prepare a spiking solution in methanol or acetone thatcontains Arolclor 1016 and Aroclor 1260 at a concentration of 10 pg/mL. Depending on theproject, another Aroclor may be used for the matrix spike solution. Sodium hydroxide (NaOH), 10 Normal (N)-Weigh out 40g ofNaOH and dissolve in 100mL ofdeionized water. Sulfuric acid, concentrated Sulfuric acid (HzSO4), 1:1-Add and equal volume of concentrated H SO4 to an equal volume ofdeionized water. Deionized (DI) water, Type II or equivalent SOP: 1801 PAGE: 9 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 7.0 PROCEDURES 7.1 Sample Preparation and Extraction for Water 1. Transfer the sample container into a fume hood. Mark the meniscus of the sample levelwith an indelible marker, and pour the sample into a 2-liter separatory funnel. Check thepH of the sample with wide range pH paper and record it in the extraction log. Ifthe pH isnot neutral, adjust the pH between 5 and 9 with 10 N sodium hydroxide and/or 1:1 sulfuricacid solution. Pour the tap water into the sample bottle to the meniscus line. Measure thetap water volume using a 1 liter graduated cylinder and record the volume on the extractionlog. 2. Prepare a method blank and laboratory control sample (LCS) by transferring 1-L of DIwater into a 2-L separatory funnel. A method blank and LCS must be prepared for every20 samples or per batch. 3. Measure two additional 1-L portions of sample for use as a matrix spike and matrix spikeduplicate (MS/MSD) at a rate of one MS/MSD per every 10 samples or 10%. NOTE::This sample may be specified on the chain of custody record for this purpose bythe SERAS Task Leader. 4. Add 1 mL of the 200 nanograms/milliliter (ng/mL) surrogate working solution to themethod blank, LCS, MS/MSD and all the samples or add sufficient volume to result in afinal concentration of200 parts per billion (ppb) in the final extract. 5. Add 100 pL of the 10 pg/mL Aroclor spiking solution to the LCS and MS/MSD or addsufficient volume to achieve a final concentration of 1.0 parts per million (ppm) in the finalextract if a higher extract volume will be obtained. 6. Rinse the sample bottle with 60 mL of methylene chloride, transfer the rinsate to theseparatory funnel and extract the sample by shaking the funnel for two minutes, withperiodic venting to release excess pressure. Allow the organic layer (generally the bottomlayer) to separate from the water phase. If the emulsion interface between layers is morethan one-third the volume of the solvent layer, the extraction chemist must employmechanical techniques to complete the phase separations. The optimal techniquesemployed depend upon the sample, and may include stirring, filtration of the emulsionthrough glass wool, centrifugation, or other physical means. Ifusing a bench top shaker,vent and release excess pressure, place it on shaker and shake for 5 minutes. 7. Filter the extract (bottom layer) through a funnel containing glass wool and anhydroussodium sulfate into a 500-mL Erlenmeyer flask.Add a second 60-mL portion ofmethylene chloride to the separatory funnel and repeat the extraction procedure a secondtime, combining the extracts in the Erlenmeyer flask. Perform a third extraction in thesame manner. After the third extraction, rinse the sodium sulfate in the funnel with SOP: 1801 PAGE:10 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENTSAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) sufficient methylene chloride. If using the bench top shaker, shake the sample(s) for 3minutes for the 2nd and 3d extractions. 7.2 Sample Preparation and Extraction for Soil 1. In a fume hood, place 140 mL of 1:1 acetone/hexane into aSoxtherm eexxttrraaccttion vesselcontaining 2 boiling chips. If using Soxhlet extraction, place 300 mL of 1:1acetone/hexane in a 500-mL round bottom flask containing 3 or more clean boiling chips. 2. Transfer the sample container into the fume hood. Open the sample bottle and discard anyforeign objects such as sticks, leaves, and rocks. Mix the sample thoroughly. 3. Calibrate the balance with Class “S” Weights prior to weighing samples or on a daily basiswhen the balance is in use.The balance should be calibrated with a weight that is similarto the weight used to extract the samples (i.e.,30 g). 4. Weigh approximately 30 g of each sample to the nearest 0.1g into a glass container and adda sufficient amount (approximately 30-100g) of anhydrous granular sodium sulfate. Mixwell. The sample should have a sandy texture at this point. A method blank and LCSmust be prepared by using 30 g of sand (or baked sodium sulfate) according to the sameprocedure as the samples, at the frequency of one per 20 samples or per batch. 5. Transfer sample to a pre-cleaned extraction thimble.Place the thimble containingsamples to extraction beaker. For Soxhlet extraction, take a piece of baked glass wool andplace it in the Soxhlet extractor so it covers the bottom of the inner diameter. Add somesodium sulfate to hold the glass wool in place; this will prevent any soil/sediment frombeing caught and clogging the Soxhlet. Add the blended sample and anhydrous sodiumsulfate into the Soxhlet extractor on top of glass wool and sodium sulfate. 6. Weigh two additional 30 g portions of the sample chosen for spiking to the nearest 0.1 g foruse as a MS/MSD at a rate of one per ten samples per project, or ten percent. NOTE::This sample may be specified on the Chain of Custody record for this purpose bythe SERAS Task Leader. 7. Add 0.5 mL of the 2 ug/mL surrogate working solution to the method blank, LCS,MS/MSD and all the samples or add sufficient volume to result in a final concentration of200 ppb in the final extract. 8. Add 0.5 mL of the 10 ug/mL matrix spiking solution to the LCS and MS/MSD or addsufficient volume to achieve a final concentration of 1.0 ug/mL in the final extract. 9. Attach the extraction vessel to the Soxtherm Extractor and extract for 2 hours.TTheSoxtherm extraction conditions are specified at Appendix C. For Soxhlet extraction,attach condenser to the extractor and flask, and extract the sample(s) for 16 hours. SOP: 1801 PAGE:11 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) NOTE: Caremust be taken to supervise the beginning of the extraction to ensure that thecondenser is cooling the evaporating solvent sufficiently to guarantee that the solvent willcondense and continue to extract the sample in a continuous cycle for the entire extraction.Allow the extract to cool after the extraction is complete. 7.3 Sample Concentration for Water and Soil Extracts 1. If concentrating using a TurboVap apparatus, skip to step 5. Otherwise, assemble aKuderna-Danish (K-D) apparatus by attaching a 10-mL concentrator tube to a 500-mLevaporation flask. Transfer the extract to the K-D concentrator 2. Add one or two clean boiling chips to the evaporation flask and attach a three-ball Snydercolumn. Place the K-D apparatus on a hot water bath (70 to 75 C) so that the concentratortube is partially immersed in the hot water and the entire lower rounded surface of the flaskis bathed with hot vapor. Addapproximately 1 mL of hexane to the top of Snyder column.Adjust the vertical position of the apparatus and the water temperature as required tocomplete the concentration. At the proper rate of distillation, the balls of the column willactively chatter, but the chambers will not flood with condensed solvent. When theapparent volume of liquid is below 10 mL, add another 60 mL of hexane and evaporatedown to below 10 mL. Remove the K-D apparatus, and allow it to drain and cool. NOTE:DO NOT ALLOW THE EXTRACT TO GO TO DRYNESS. IHowever, if theextract goes to dryness, document the situation in the extraction logbook. 3. Remove the Snyder column; use 1-2 mL of hexane to rinse the flask and its lower joint intothe concentrator tube.Remove the concentrator tube and place it onto the N-Evappreheated to 35 C. 4. Evaporate the extract to a final volume of 1 mL for water and 5 mL for soil. Duringevaporation, rinse the wall of the concentrator tube with 1-2 mL of hexane. Continue withstep 7. NOTE::1DO NOT ALLOW THE EXTRACT TO GO TO DRYNESS. IHowever, If theextract goes to dryness, document the situation in the extraction logbook. 5. If using the TurboVap concentrator, fill the TurboVap water bath with approximately onegallon of deionized water mixed with 10-15 drops of Clean Bath solution. Set the waterbath temperature at 55 C. 6. Transfer the extract to the concentrator cells in the hood. Begin concentrating by blowinga gentle stream of nitrogen into the cells so that no solvent is splashed out. As the solventlevel is reduced, add any remaining extract, rinse the flask with hexane, and add the rinsateto the concentration cell. Once all the extract has been transferred to the concentrator celland the solvent level is well below the 200-mL mark, the flow of nitrogen can be increasedto speed up the concentration. Periodically rinse the cell with hexane. Concentrate theextract below 10 mL, add 60 mL of hexane and concentrate it down to a final volume of SOP: 1801 PAGE:12 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENTSAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 1mL of water and 5 mL for soil. NOTE: DO NOT ALLOW THE EXTRACT TO GO TO DRYNESS. However, If theextract goes to dryness, document the situation in the extraction logbook. 1. Take a 4-mL aliquot of the sample extract from Steps 4 or 6 and proceed to the optionalGPC cleanup for soil in Section 7.5, optional Florisil cleanup described in Section 7.6,optional TBA-sulfite cleanup described in Section 7.7 or optional copper cleanupdescribed in Section 7.8, and sulfuric acid cleanup described in section 7.9. Store theremaining extract(s) at 4 C±2 C. Note: Record the date and the applicable samples subjected to cleanup on the extractionlog. 7.4 Total Solids The sample aliquots for total solids are weighed in conjunction with the samples for the extraction.The total solids for the MS/MSD are based on the corresponding sample. The blank is assumed100% total solids. Weigh and record (in the % solid logbook) an empty aluminum sample dish to the nearest 0.01 g.Weigh at least 10 g of the soil/sediment into the aluminum dish and record the weight. Preheat theoven to 103-105C. Place the aluminum dishes in the oven and record the initial temperature inthe logbook. Determine the percent total solids by drying the sample(s) in an oven that is placedinside a fume hood overnight. In the morning, record the final temperature of the oven in thelogbook.. Turn off the oven and allow the dishes to cool in the desiccator before weighing.Concentrations of individual analytes will be reported relative to the dry weight of the soil orsediment. Calculate the percent total solids using the following equation. 7.5 Gel Permeation Chromatography Cleanup 1. Calibrate the GPC instrument by injecting 10 pL of GPC standard (corn oil, bis(2-ethylhexyl) phthalate, methoxychlor, perylene, and sulfur)and eluting it with methylenechloride to establish collection time window to collect the fraction from the beginning ofethoxychlor peak to the end of perylene peak. 2. When the collection time window has been established, inject a methylene chloride blankto make sure all calibration components are washed from the column. 3. Before injecting the samples, dilute the 1 mL water extracts to 4 mL including methodblanks, LCSs and MS/MSDs with methylene chloride. Filter the 4 mL of each extractthrough acrodisc CR PTFE filter (Gelman, 0.45pm) into a clean 4-mL vial. Load the 4-mL vials, which contain pre-filtered extracts onto the autosampler and start thesequence. SOP: 1801 PAGE:13 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) Collect the cleaned extracts from the fraction collector, transfer to the concentrator tubesand concentrate the extracts to a final volume of 2 mL using TurboVap. Weight of Dried Sample with Dish (g)%Total Solids=- Dish Weight(g)Weight ofWet Sample with Dish (g)-Dish Weight(g) NOTE: GPC Pump flow rate is 5.0 mL/minuteGPC Run time is 25 minutes 7.6 Florisil Cleanup Florisil cleanup significantly reduces matrix interferences caused by polar compounds. 1. Place one Florisil cartridge into the manifold for each sample extract to be subjected tocleanup. 2. Prior to the cleanup of samples, the cartridges must be washed with 90:10 hexane/acetone.This is accomplished by passing through at least 10 mL of the hexane/acetone solutionthrough each cartridge. NOTE: DO NOT ALLOW THE CARTRIDGES TO DRYAFTER THEY HAVE BEEN WASHED. 3. After the cartridges in the manifold are washed, a rack containing labeled 25-mLconcentrator tubes is placed inside the manifold. Care must be taken to ensure that thesolvent line for each cartridge is placed inside of the appropriate concentrator tube as themanifold top is replaced. 4. After the concentrator tubes are in place, add approximately 1 mL of the 90:10hexane/acetone solution to the Florisil bed in the cartridge. Allow the solvent to pass intothe sorbent bed and immediately transfer 2 mL from each sample, blank and MS/MSDextract from Section 7.3, Step 7 to the top of the Florisil bed in the appropriate Florisilcartridge. 5. The extracts are then eluted through the cartridge with 18 mL of 90:10 hexane/acetone andcollected in 25-mL concentrator tubes held in the rack inside the manifold..1NOTE: Besure to add the 18 mL of mobile solution immediately after the 2-mL extract crosses theFlorisil bed. 6. Transfer the concentrator tubes from step 5 to the TurboVap LV and concentrate theextracts to a final volume of 2 mL using nitrogen blow down. 7.7 Tetrabutylammonium (TBA)-Sulfite Cleanup Elemental sulfur is encountered in many soil/sediment samples..The solubility of sulfur in theextraction and exchange solvents is very similar to the multi component PCBs;therefore, the sulfuris extracted along with the PCBs. If the GC is operated under normal conditions for PCB analysis, SOP: 1801 PAGE:14 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) the sulfur interference can completely mask the region from the solvent peak through PCB patterns.This cleanup is used to remove the sulfur interference. 1. Transfer the extract from Section 7.3, Step 4 or Section 7.5, Step 5, to a 25-mL test tube. 2. Add 2 mL ofTBA-sulfite reagent and 2 mL of 2-propanol; cap and shake vigorously with amechanical shaker such as Vortex for at least two minutes. If the sample is colorless or ifthe initial color is unchanged, and if clear crystals (precipitated sodium sulfite) areobserved, sufficient sodium sulfite is present. If the precipitated sodium sulfitedisappears, add more TBA-sulfite reagent until a solid residue remains after repeatedshaking. 3. Add 6 mL of deionized water and shake for at least two minutes.S.Allow the sample tostand for 5-10 minutes.Transfer the hexane layer (top) to two 1-mL injection vials. 7.8 Copper Cleanup Copper cleanup requires that the copper powder be very reactive. Transfer the sample extract from Section 7.3, step 7 or Section 7.5, step 5 or Section 7.6,step 6 to a 4-mL screw-top vial. 2. Add approximately 0.5 to 2 g of copper powder (depends on the color and viscosity ofsample) to the vial. Vigorously mix the extract and copper powder for at least 1 minute ona mechanical shaker such as Vortex. Allow the copper to settle. 3. Separate the extract from the copper by drawing off the extract with a disposable glasspipette into two 1-mL injection vials. 7.9 Sulfuric Acid Cleanup Rigorous sulfuric acid cleanup is suitable for the sample extracts of PCBs.. A1cid cleanup must beused whenever elevated baselines or overly complex chromatograms prevent accurate quantitationofPCBs. 1. Transfer 2 mL of sample extract from Section 7.3 step 7 or Section 7.5, step 5 or Section7.6 step 6, or Section 7.7 Step 3 to a 4-mL screw-top vial. 2. Add 2 mL of concentrated sulfuric acid and cap the vial tightly and vortex for one minute.A vortex must be visible in the vial. 3. Allow the phases to separate for at least 1 minute.Transfer the top (hexane) layer into theinjection vial for analysis on GC/ECD. Ifthe extract is colored, repeat the step for 2"dand3" time by transferring the hexane layer to another 4-mL vial. 4. If there is separation problem, a centrifuge can be used to separate the layers. SOP: 1801 PAGE:15 of37 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 7.10 GC/ECD Conditions Sample analyses are performed using a Hewlett Packard (HP) 6890 GC/ECD, equipped with dualinjector, column, and electron capture detector capabilities. The HP 6890 conditions used for the PCB analysis are listed below: Injector Temperature250°COven Temperature Program120°C hold for 1 minute (min)9°C/min to 285°℃, 10 min at 285℃Detector Temperature300°CCarrier GasHeliumMake-up GasArgon/MethaneColumn Flow RateRTX-XLB 3.0 milliliters/minute (mL/min);RTX-CL Pesticides 1 mL/minAmount Injected1 microliter (uL)Data SystemHP Chem Station The instrument conditions listed above are guidelines to be used for standards and sample analysison a HP 6890 GC/ECD system. Any suitable conditions may be used as long as quality controlcriteria and peak separation is achieved. 2 7.11 Retention Time Windows Due to advances in electronic pressure controls in modern GCs such as the HP6890, the RTs usuallyremain constant and may exhibit a negligible shift (nearly zero) over the traditional 72-hour period.A default standard value of±0.030 minutes will be used for the two surrogates and a value of±0.020minutes will be used for the internal standards.These default values will be applied unless theinstrument and EPC unit cannot maintain constant retention times. If the instrument cannotmaintain reproducible RTs, the analyst must investigate the cause and implement corrective action 7.12 Standard and Sample Analysis The analytical sequence listed in Figure 1, Appendix B must be followed. 1. Prepare calibration standards as in Section 6.0. For the initial determination of PCBpresence in a sample extract, inject 1 uL each of the five PCB 1016/1260 workingcalibration standards. Choose five peaks (peaks must be >25% of full scale) to calculateresponse factors (RFs). Once the type of Aroclor is known or suspected, inject 1 uL eachof the five working calibration standards for those Aroclors.. The average RF and percentrelative standard deviation (%RSD) must also be calculated for each Aroclor peak on thecolumn used for quantitation, using the equations below. SOP: 1801 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) where: Ax=IPeak Area or Peak Height of each target analyte As=IPeak Area or Peak Height of each internal standard assignedto target analytes Cis =Concentration of each internal standard (ng/mL) Cx = Concentration of each target analyte (ng/mL) 2. External Standard Method Prepare calibration standards as in Section 6.0. For the initial determination of PCBpresence in a sample extract, inject 1 uL each of the five PCB 1016/1260 workingcalibration standards and tabulate the peak height or peak area for each standardconcentration. Calculate the response factor (RF) for each compound at each standardconcentration using the equation below. Once the type of Aroclor is known or suspected,inject 1 uL each of the five working calibration standards for those Aroclors. The averageRF and percent relative standard deviation (%RSD) must also be calculated for bothcolumns using the equations above in Section 7.9.1 Step 3. The%RSD (average of all 5 peaks) for each Aroclor must be ≤20.0% for the internalstandard method and external standard method. If analytes are reported from bothcolumns, the %RSD must be supplied for both columns. NOTE: An initial calibration curve must be run every six months at a minimum or soonerif the daily calibration check doesn’t meet the required percent difference (%D) as SOP: 1801 PAGE:17 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) specified in Section 7.12, Step 4. NOTE: If a sample extract is initially run for pesticides/PCBs and it is determined that noPCBs are present, a PCB initial calibration will not be run. 3. Inject 1 ppm each of the remaining Aroclors (Ar), Ar 1016, Ar 1232, Ar 1242, Ar 1248, Ar1254, Ar 1260, and Ar 1268 and 2 ppm of Ar 1221 for the fingerprints. NOTE: The fingerprints are used for qualitative pattern matching only..The fingerprintsneed not be run with each initial calibration curve as long as the date of the fingerprints donot exceed one year or a new source of standards is received. 4. For every 12 hours of sample analysis, inject the 1 ppm mid-point calibration standard.Calculate and tabulate the %D for each Aroclor peak using the following equation: INT where: ARFINTr == IInitial Average Response FactorRFCALC = (Calculated Response Factor Note: the %D for the Daily Check standard must be ≤15% 5. Inject a group of sample extracts.IIt is recommended to inject the method blank and LCSfirst. All sample extracts must be analyzed within 12 hours of the injection of the 1 ppmcontinuing calibration Aroclor standard (step 4). 6. Repeat steps 4 and 5, if necessary, until the %D requirement of the continuing calibrationcheck fails or the sample sequence is complete. 7.13 Evaluation of Chromatograms All standard and sample chromatograms must be evaluated to determine if re-injection and/ordilution is necessary. 7.13.1 Standard/Sample Chromatograms The following requirements apply to all data presented for multi component analytes. 1. The PCB chromatograms must display the multi-component analytes present in eachstandard or sample at greater than () 25% and less than (<)100% of full scale. Thechromatogram must be printed in landscape mode with the time scale of thechromatogram from approximately 5 (before the TCMX) to 25 minutes (after theDCBPpeak). IIf the time scale is modified, all chromatograms will be scaled the same SOP: 1801 PAGE:18 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT. SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) for comparative purposes. 2. If an extract is diluted, chromatograms must display multi-component PCBs between10% and 100% of full scale. 3. If a chromatogram is re-plotted electronically to meet requirements, the scaling factorused must be displayed on the chromatogram 4. If a chromatogram indicates carryover from a previous injection, subsequent sampleextract(s) must be re-analyzed, preferably immediately. 5. The retention time of each surrogate and internal standard must fall within the RTwindow criteria in Section 7.10. Ifthe RT window has shifted in the thousandth place(>0.030 but <0.040), professional judgment may be used to determine theacceptability of the data. If the RT is shifted by more than 0.040 minutes, theanalytical sequence (acquisition) must be interrupted for corrective action. Aftercorrective action, acquisition of data can be resumed only after an acceptable 1 ppmPCB standard is obtained. No retention time windows are required for PCBs analysissince the identification of PCBs is done by pattern recognition. 6. If a sample chromatogram has interfering peaks, a high baseline, or off-scale peaks,the sample extract must be re-analyzed using a dilution, further cleanup, orre-extraction. Samples that do not meet acceptance criteria after one re-extractionand cleanup must be reported in the case narrative and do not require further analysis. 7. A sample which under going additional cleanup must also include the method blank 8. If it is determined that the matrix may be causing a RT shift, the internal standard andthe MS/MSD in conjunction with the original sample chosen for spiking may be usedto assess matrix effects. 9. If maniuuaall integrations have been performed, refer to SERAS SOP #1001,Chromatographic Peak Integration Procedures for appropriate documentation. 7.13.2 PCB Identification The identification ofPCBs is based on pattern recognition, which can only be verified froman on scale chromatogram. Second column confirmation is optional for PCB analysis. 7.14 Sample Dilution Target compound concentrations must not exceed the upper limit of the initial calibration range.Ifanalytes are detected in the extract at a level greater than the highest calibration standard, the extractmust be diluted (to a maximum of 1:100,000) or until the analyte response is within the linear rangeestablished during calibration. Guidance in performing dilutions and exceptions to this SOP: 1801 PAGE:19 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) requirement are given below. 1. Ifthe analyst has reason to believe that diluting the final extracts will be necessary based onhistorical data or visual observation of the extracts, an undiluted run may not be required.However, if no peaks are detected above 25% of full scale on the diluted sample, analysisof a more concentrated sample extract or the undiluted sample extract is required. 2. Ifthe response is still above the highest calibration point after the dilution of 1:100,000, theanalyst should contact the Organic Group Leader immediately for further instruction. 3. The results of the original analysis are used to determine the approximate dilution factorrequired to bring the largest analyte peak within the initial calibration range. 4. The dilution factor chosen should keep the response of the largest peak for a targetcompound in the initial calibration range of the instrument. 5. Submit data for any reportable analyses. 6. All chromatograms for the dilution analyses must meet the requirements described inSection 7.13. 8.0 CALCULATIONS Reporting of target compounds and surrogates can be performed on any column that passed all the qualitycontrol (QC) criteria specified in this SOP. In order to be quantitated, the detector response (peak area orpeak height) of all the analytes must lie within the calibration range. 8.1 Reporting Limit for Water where: CSTD 一 Concentration of the lowest standard in thecalibration range (ug/mL) Vr Volume of the extract (mL) DF Dilution factor Vo Volume of water extracted The typical RL for each analyte can be found in Appendix A. 8.2 Reporting Limit for Soil SOP: 1801 PAGE:20 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) RL (u/kg)dryweightCsmoF= where: CSTD = Concentration of the lowest standard in thecalibration range (ug/mL) Volume of the extract (mL) Dilution factor :Volume of water extracted (L) S Decimal Percent Solids The typical RL for each analyte can be found in Appendix A. 8.3 Sample Concentration for Water Using Internal Standard Method Use the following equation to calculate the concentration of the identified analytes using the relativeresponse factor (RRF) obtained from the initial calibration curve. where: Peak Area or Peak Height of each target analyte Amount of each internal standard injected (ng) Volume of the concentrated extract (mL) DF Dilution factor Ais Peak Area or Peak Height of each internal standard RRFavg= Average Relative response factor Volume of water extracted (L) Injection volume (usually 1 pL) 8.4 Sample Concentration for Water Using External Standard Method where: Peak area or peak height for the compound to be measured Volume of the concentrated extract in microliters (uL) Dilution factor RFAvG=Average response factor Vo Volume of water extracted in milliliters (mL) SOP: 1801 PAGE:21 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENTSAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 二 Volume of extract injected in microliters (uL) 8.5 Sample Concentration for Soil Using Internal Standard Method Identified target analytes will be quantitated by the internal standard method.1. The internal standardused must be the one nearest the retention time to that of the given analyte listed in Table 2,Appendix A.Use the following equation to calculate the concentration of the identified analytesusing the relative response factor (RRF) obtained from the initial calibration curve. where: A= Peak Area or Peak Height of each target analyteA Amount of each internal standard injected (ng) Volume of the concentrated extract (mL) Dilution factor Peak Area or Peak Height of each internal standard RRFavg= Average Relative response factor WWeight of soil/sediment extracted (kg) S V Decimal percent solid Injection volume (usually 1 pL) 8.6 Sample Concentration for Soil Using External Standard Method Unusual sample matrices which interfere with the internal standards, and/or unusual circumstancesduring analysis may warrant the use of the external standard method at the discretion of the OrganicGroup Leader. where: 4x =Peak Area or Peak Height for the compound to bemeasured =Volume of the concentrated extract(mL) DF =TDilution factor (ifany) RFAvG =Average response factor =\Weight of soil/sediment extracted (kg) S Volume of extract injected (uL) =IDecimal percent solids NOTE:: If any analytes are detected below the quantitation limit at>25% of the quantitation limit,report the concentration and flag as estimated (J). SOP: 1801 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 8.7 Surrogate Spike Recoveries Percent Recovery (%R)=2Dx100 04 where: =Cquantity determined by analysisQ=cquantity added to sample 8.8 Matrix Spike Recoveries The percent recoveries and the relative percent difference (RPD) between the recoveries of each MSand MSD are calculated and reported using the following equations: SA where: SSR=: spike sample result SR= sample result SA=sspike added where: RPD=Relative Percent Difference MSR = matrix spike recovery MSDR = matrix spike duplicate recovery The vertical bars in the formula above indicate the absolute value of the difference; hence, RPD isalways expressed as a positive value. 8.9 Laboratory Control Sample Recoveries The recoveries of each of the compounds in the LCS solution will be calculated using the followingequation: SA where: SOP: 1801 PAGE:23 of37 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENTSAMPLES BY GC/ECD (EPA/SW-846 Methods3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) LCSR = Concentration of target analyte in LCS B = Concentration of target analyte in blank SA =Concentration of spike added 8.10 Dixon's Criterion Dixon’s criterion at the 95% confidence level is used to identify statistical outliers in a data. Thistest should be applied sparingly and never more than once to a single data set. FFor weatheredsamples, all peaks are included in the quantitation even if any of the peaks fail Dixon’s criterion. To determine if a peak may be omitted from the average of the peaks used for quantitation,arrangethe results in ascending order (X1,X2,.......Xn).J.If X, is to be tested as an outlier,use the followingequation: Reject Xn as an outlier if the ratio is greater than Dixon’s Criterion for n (see Table 2, Appendix A)If X is to be treated as an outlier, use the following equation: Reject Xas an outlier if the ratio is greater than Dixon’s Criterion for n (see Table 2, Appendix A). 9.0 QUALITY ASSURANCE/ QUALITY CONTROL 9.1 Holding Time Extraction of soil/sediment samples must be completed within 7days of sampling, and analysiscompleted within 40 days of sample extraction. 9.2 Identification of Target Compounds The identification of multi-component PCBs is based primarily on pattern recognition; secondarycolumn confirmation is optional. 1. If<3 peaks are used in quantitation, the analyst will report the result as estimated and flagas“J”. Document in the case narrative the reason why the original five peaks were notused for the quantitation. 2. For samples in which several Aroclors can be identified as present and quantitation wouldbe difficult due to overlapping peaks with other Aroclors, the analyst will note theinterference in the case narrative and report data based on the major Aroclor(s) present. SOP: 1801 PAGE:24 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) The Organic Group Leader will discuss whether the lower concentration Aroclor(s) is (are)significant for the project with the SERAS Task Leader. 9.3 Initial Calibration for Target Compounds and Surrogates For the initial determination of PCBs, prior to the analysis of any sample, method blank, LCS orMS/MSD, the GC/ECD system must be initially calibrated with a minimum of five 1016/1260working calibration standards to determine the linearity range. Ifreporting data from only onecolumn, the %RSDs will be reported from only that column. Ifreporting data from both columns,all corresponding calibrations will be required for both columns. Once the type of Aroclor isknown or suspected, the GC/ECD system must be calibrated with a minimum of five workingcalibration standards for the particular Aroclors known or suspected to be present to determine thelinearity range. 1. The concentration of all calibration standards that are specified in Section 6.0 must beused. 2. The standards are to be analyzed according to the procedures given in Section 7.12 usingthe GC operating conditions in Section 7.10. 3. The response factors are determined according to the procedure in Section 7.12. 4. The initial calibration is also evaluated on the basis of the stability of the response factorsof each target compound and surrogate. The average %RSD of each five peak must andthe two surrogates must not exceed 20.0%. 9.4 Continuing Calibration for Target Compounds and Surrogates Once the GC/ECD system has been calibrated, the calibration must be verified each 12-hour timeperiod during which samples are analyzed. Ifreporting data from only one column, the %D will bereported only for that column.1。Ifreporting data from both columns, all corresponding %Ds will berequired for both columns. 1. The %D for the average of five peaks and surrogates must not exceed 15.0%. 2. The continuing calibration is evaluated on the GC column used for analysis in theinstrument. 3 If the %D exceeds 15.0% for a specific analyte and the analyte is present in the sampleextract, re-injection is required to ensure an accurate concentration. 4. If the end of sequence (EOS) exceeds 15.0% for the average of the 5 peaks, the samplesmust be re-analyzed. If the EOS does not meet acceptance criteria after re-analysis, itmust be documented and further analysis is not required if the failing compounds are notdetected in the associated samples. The analytical sequence may be altered on thereanalysis by introducing additional calibration check standards at a greater frequency. SOP: 1801 PAGE:25 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 9.5 Retention Time Windows A default standard value of ±0.030 minutes will be used for the two surrogates. Since theidentification of multi-component PCBs by GC methods is based primarily on pattern recognition ofa group of peaks, specific retention time windows are not used for the individual PCB peaks. 1. The retention time shifts of the surrogates and internal standard may be used to evaluate thestability of the GC system during the analysis of standards only. 2. Retention time windows must be established on both columns for the surrogates andsubmitted with the data package. 9.6 Analytical Sequence The standards and samples analyzed by this SOP must be analyzed in the sequence outlined inFigure 1, Appendix B. This sequence includes requirements that apply to the initial and continuingcalibrations, as well as the analysis of samples. 9.7 Method Blank and Laboratory Control Sample Analysis A method blank and LCS are a known volume of deionized water or a known weight of a cleanreference matrix (pure sand or baked sodium sulfate) that is carried through the entire analyticalprocedure. The volume or weight used for the method blank must be approximately equal to thevolume or weight of the samples associated with the blank. The purpose of the method blank is todetermine if there are any contaminants associated with the processing and analysis of samples. 1. For PCB analysis only, a method blank and LCS must be prepared for each group of up to20 samples extracted at the same time or per batch and analyzed on each GC/ECD systemused to analyze samples. NOTE: If a sample has been extracted for pesticides/PCBs, the LCS will contain pesticidecompounds only. 2. A method blank must not contain any of the compounds listed in Table 1, Appendix A atconcentrations greater than or equal to () the reporting limit. 3. The method blank must be analyzed on all the instrument on which samples were analyzed. 4. All samples associated with an unacceptable method blank must be re-extracted andre-analyzed if sufficient volume or mass is available and the holding time has not beenexceeded.Otherwise, it will be documented in the case narrative of the data package. 5. When sample extracts are subjected to cleanup procedures, the associated method blankmust also be subjected to the sample cleanup procedure. SOP: 1801 PAGE:26 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 6. Method blank results must not be subtracted from any associated samples. Use 70 to 130% for LCS recoveries until ranges are established. LCS %recovery less than 70% must be reported tothe Organic Group Leader.A solvent blank may be used to check for contamination or carryover from a previoussample. If an analyte present in the solvent blank is found in subsequent samples, the samples should be re-analyzed.A solvent blank may also be run prior to any standard 9.8 Surrogate Recoveries 1. Surrogates are added to each sample, blank, LCS, MS, and MSD prior to extraction at theconcentrations described in Sections 6.0 and 7.1. 2. The surrogate spike recoveries are calculated according to the procedures in Section 8.7. 3. The quality control limit for both surrogate recoveries is 30-150%. These limits are onlyadvisory, and no further action is required if the limits are exceeded. However, frequentfailures to meet the limits for surrogate recovery warrant investigation by the laboratory. 9.9 Internal Standards 1. Add 20 uL of 5 ppm internal standard to all standards and samples including the lab blanks,LCSs and MS/MSDs or add sufficient amount to achieve a final concentration of 100 ug/L. 2. The peak height (or area) ofthe internal standards in each sample must be monitored bythe analyst and to assure the height (or area) falls between 50% and 150% of thecorresponding internal standard in the daily calibration check.. If any the internalstandards do not meet the criteria (50% to 150%), then it will be flagged with (*). Note: Only the internal standards used to calculate the target compounds will be monitored 3. If one or more internal standard areas do not meet criteria, the GC system must beinspected for malfunctions and corrections made as appropriate. When corrections aremade, re-analysis of all affected samples is required. Ifre-analysis is not feasible due tomatrix interference (i.e., coeluting with IS peak) on both columns, the analyst may chooseto dilute the sample to remove the interference instead of re-analyzing. 4. If after re-analysis, the areas for all internal standards meet criteria (between 50% and150%), then the problem with the first analysis is considered to have been within thecontrol of the laboratory. Therefore, only data from the analysis with the ISs within limitsare required to be submitted. Ifre-analysis confirms matrix effects, submit both sets ofdata but report the initial run 9.10 Matrix Spike and Matrix Spike Duplicate Analysis The purpose of spiking target compounds into two aliquots of a sample is to evaluate the effects ofthe sample matrix on the methods used in this SOP. SOP: 1801 PAGE:27 of 37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 1. The MS/MSD must be prepared with every 10 samples per matrix or per project,whichever is more frequent. 2. For PCB only analysis, the matrix spiking solution specified in Section 6.0 must be usedand result in the concentration specified in Sections 7.1 and 7.2. NOTE: If a sample has been extracted for pesticides/PCBs, the MS/MSD will containpesticide compounds only. 3. The MS/MSD recoveries and the % RPD are calculated according to the procedures inSection 8.8.There are no acceptance limits for the PCB MS/MSD recoveries. 4. Dilution and re-analysis of the MS/MSD is required to bring the target analytes within thelinear range of the calibration curve.Dilution and re-analysis of the MS/MSD is notperformed for non-target analytes outside the calibration range. 5. The MS/MSD must be extracted and analyzed within holding time. 9.11 Initial Demonstration of Capability Initial proficiency in PCBs analysis must be demonstrated by each analyst initially and each timesignificant changes are made in the procedure or for instrumentation. Each analyst will generateprecision and accuracy data using a reference standard other than the source used for calibration.Four replicate of a well-mixed reference standard is analyzed using the procedures outlined in thisSOP. Calculate the average mean in ug/kg for soil and pg/L for water and the standard deviation(S) in pg/kg for soil and ug/L for water.. The QAO will tabulate the results from all of the analystsper matrix per parameter, and calculate control limits. 9.12 Dilution Analysis 9.13 If the concentration of any sample extract exceeds the initial calibration range, that sample extractmust be diluted and re-analyzed as described in Section 7.12. If there are no peaks detected above25% ofthe full scale in the dilution analysis, a lower dilution of the sample extract must be analyzed.Reporting Limit The lowest concentration of the calibration standard that is analyzed during the initial calibrationdetermines the method reporting limit based on the initial volume or weight of the sample, finalvolume of extract obtained from the extraction, and percent total solids, if appropriate. 9.14 Method Detection Limit Studies Method detection limit (MDL) studies will be run on an annual basis for the water and soil matrix toverify the minimum concentration that can be measured and reported with 99% confidence. Aminimum of seven replicates will be used for the study (EPA 1984). SOP: 1801 PAGE:28 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 9.15 Nonconformance Memo A nonconformance memo will be generated any time an employee notices a deficiency suspected ofbeing a nonconformance. This nonconformance memo will be forwarded to the QAO forverification of corrective action. 10.0 DATA VALIDATION Data will be assessed in accordance with the guidelines set forth in the most recent version ofthe SERAS datavalidation SOPs. However, data is considered satisfactory for submission purposes when ALL therequirements mentioned below are met. 1. All samples must be analyzed as part of a valid analytical sequence,i.e., an acceptableinitial calibration, and continuing calibration check at the required frequency. 2. All the QC requirements described in Section 9.0 must be met at all times. Any deviationsor anomalous conditions should be discussed with the Organic Group Leader and must bedocumented in the case narrative. 11.0 HEALTH AND SAFETY When working with potentially hazardous materials, refer to U.S. EPA, Occupational Safety and HealthAdministration (OSHA) and corporate health and safety practices. More specifically, refer to SERAS SOP#3013, SERAS Laboratory Safety Program and SERAS SOP #1501, Hazardous Waste Management. 12.0 REFERENCES National Environmental Laboratory Accreditation Committee (NELAC), Quality Systems, currentapproved version. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response. 1996.Test Methods for Evaluating Solid Waste, SW-846, 3" ed., Method 3500B. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response. 1996.Test Methods for Evaluating Solid Waste, SW-846, 3" ed., Method 3510C. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response. 1996Test Methods for Evaluating Solid Waste, SW-846, 3" ed., Method 3540C. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response. 1996.Test Methods for Evaluating Solid Waste, SW-846, 3"d ed., Method 3541. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response. 1996.Test Methods for Evaluating Solid Waste, SW-846, 3"ed., Method 3600C. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response.1996. SOP: .1801 PAGE:29 of37 REV: 2.0 DATE:01/23/06 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082)(EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) Test Methods for Evaluating Solid Waste, SW-846, 3"d ed., Method 3620B. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response. 1994.Test Methods for Evaluating Solid Waste, SW-846, 3 ed., Method 3640A. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response.1996.Test Methods for Evaluating Solid Waste, SW-846,3"ded., Method 3660B. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response. 1996.\Test Methods for Evaluating Solid Waste, SW-846, 3"ed., Method 3665A. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response..1996Test Methods for Evaluating Solid Waste, SW-846, 3 ed., Method 8000B. United States Environmental Protection Agency, Office of Solid Waste and Emergency Response. 1996Test Methods for Evaluating Solid Waste, SW-846,3"d ed., Method 8082. United States Environmental Protection Agency, Contract Laboratory Program (CLP).1999. Statementof Work for Organic Analysis, OLM04.2. United Statess EEnnvironmental Protection Agency. 1984. Federal Register, 40 Code of FRegulations (CFR) Part 136, Appendix B, Definition and Procedure of the Determination of theMethod Detection Limit -Revision 1.11, October 26, 1984. 13.0 APPENDICES A-Tables B-Figures C -Soxtherm Extractor Operating Conditions SOP: 1801 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) APPENDIX A Tables SOP #1801 January 2006 SOP: 1801 PAGE:31 of37 REV: 2.0 (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) TABLE 1..Target Compound List and Typical Reporting Limits for Water and Soil Water RL Soil RL Analyte (ug/L) (ug/kg) Aroclor 1016 0.250 41.7 Aroclor 1221 0.500 83.3 Aroclor 1232 0.250 41.7 Aroclor 1242 0.250 41.7 Aroclor 1248 0.250 41.7 Aroclor 1254 0.250 41.7 Aroclor 1260 0.250 41.7 Aroclor 1268 0.250 41.7 RL denotes Reporting Limits ' Reported on a wet weight basis SOP: 1801 PAGE:32 of37 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) TABLE 2. Target Compound List and Internal Standards 4,4'-Dibromooctafluorobiphenyl (IS) TCMX Aroclor 1016 Aroclor 1221 4,4'-Dibromobiphenyl (IS) 3,3',4,4'-Tetrabromobiphenyl (IS) Aroclor 1232 Aroclor 1242 Aroclor 1248 Aroclor 1254 Aroclor 1260 Aroclor 1268 DCBP SOP: 1801 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) 3LE 3. Dixon's Criterion for n N u m b e r o f r e s ul t s , n D i xo n ’ s Cr i t e r io n 3 0.941 4 0.765 5 0.642 6 0.560 7 0.507 SOP: 1801 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) APPENDIX B Figures SOP #1801 January 2006 SOP: 1801 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) FIGURE 1. Multi Component PCB Analytical Sequence Time Iniection # Material Injected 1-5 Initial Calibration (5-point) 6-13 PCB Fingerprints 0 hr. 14 1st sample 12 hr. n 1 ppm Continuing Calibration Standard and/or End of Sequence n+] 1st Sample 12 hr o 1 ppm Continuing Calibration Standard and/or End of Sequence Samples another 12 hr. o 1 ppm Continuing Calibration Standard and/or End of Sequence Samples another 12 hrs 1 ppm Continuing Calibration Standard and/or End of Sequence: Samples etc. last 1 ppm Continuing Calibration Standard and/or End of Sequence NOTE: All subsequent 12-hour periods are timed from the injection of the mid-point calibration standard.Theanalytical sequence must end with a continuing calibration check standard. SOP: 1801 PAGE:36 of37 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) APPENDIX C Soxtherm Extractor Operating Conditions SOP #1801 January 2006 SOP: 1801 REV: 2.0 ROUTINE ANALYSIS OF PCBs IN WATER AND SOIL/SEDIMENT SAMPLES BY GC/ECD (EPA/SW-846 Methods 3500B/3510C/3540C/3541/8000B/8082) (EPA/SW-846 Methods 3600C/3620B/3640A/3660B/3665A-Optional) Soxtherm EIxtraction Operating Conditions for PCBs ExtractionTemp. SolventVolume SolventType HotExtractionTime Evaporation A Rinsing Evaporation BInterval Evaporation C 160 140 ml hex/ace(1:1) 5x Interval TimeElapsed 50 min 25 min 30 min 0 15 min VolumeExpected 140 mL 65mL 50mL 50 mL 50mL TotalElapsedTime 55 min 80 min 110 min 110 min 120 min This method may not be changed without the expressed approval of the Organic Group Leader, the Analytical Section Leader and the Quality Assurance Officer (QAO). Only those versions issued through the SERAS document control system may be used. Modifications made to the procedure due to interferences in the samples or for any other reason must be documented in the case narrative and on a nonconformance memo.
关闭-
1/37

-
2/37

还剩35页未读,是否继续阅读?
继续免费阅读全文产品配置单
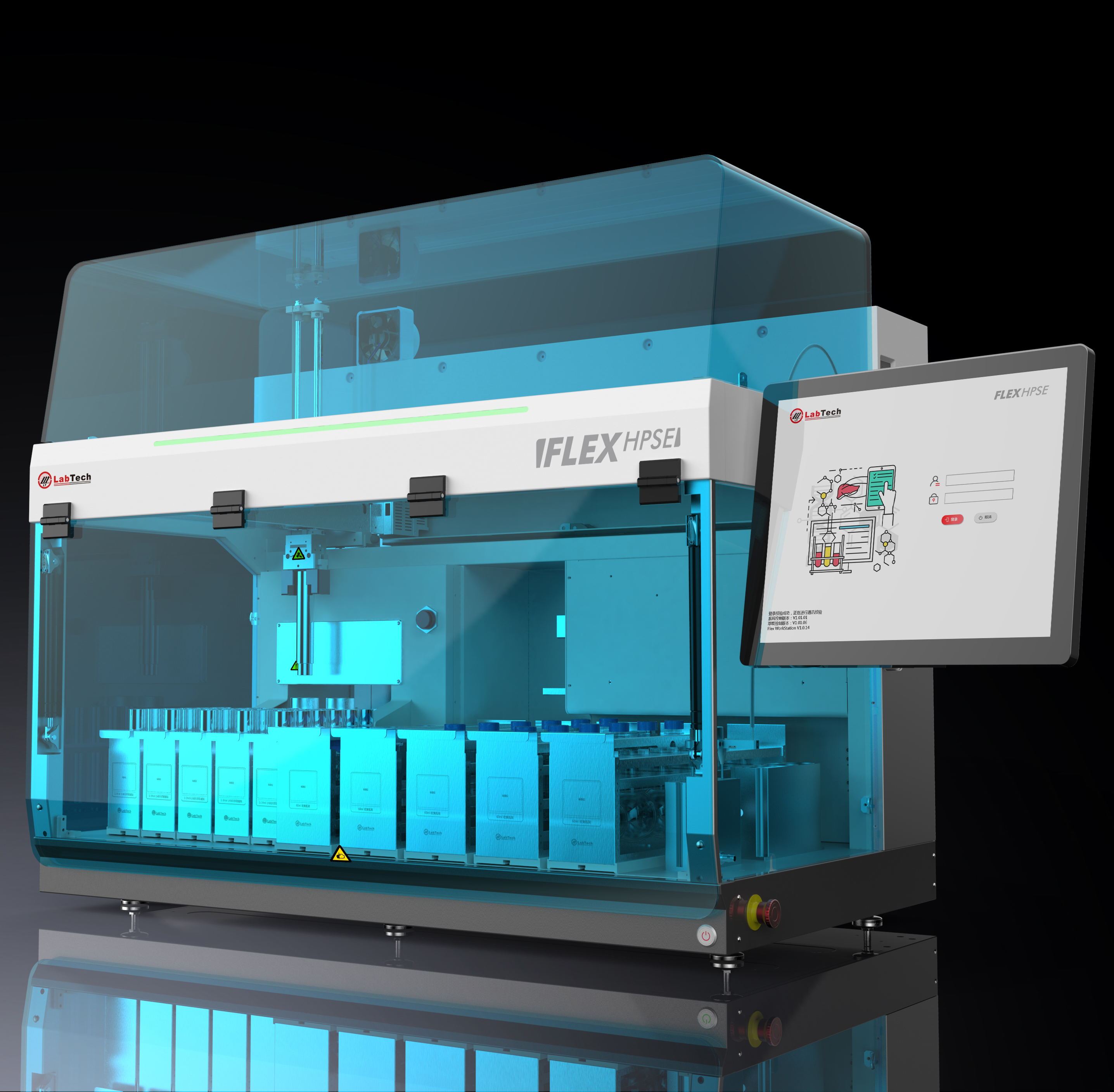
中国格哈特为您提供《水、土壤、沉积物中多氯联苯(PCBs)检测方案(快速溶剂萃取)》,该方案主要用于环境水(除海水)中有机污染物检测,参考标准《暂无》,《水、土壤、沉积物中多氯联苯(PCBs)检测方案(快速溶剂萃取)》用到的仪器有格哈特全自动快速溶剂萃取仪Sox416、德国移液器MM。
我要纠错
推荐专场
快速溶剂萃取设备/快速溶剂萃取仪
更多相关方案






 咨询
咨询





