方案详情文
智能文字提取功能测试中
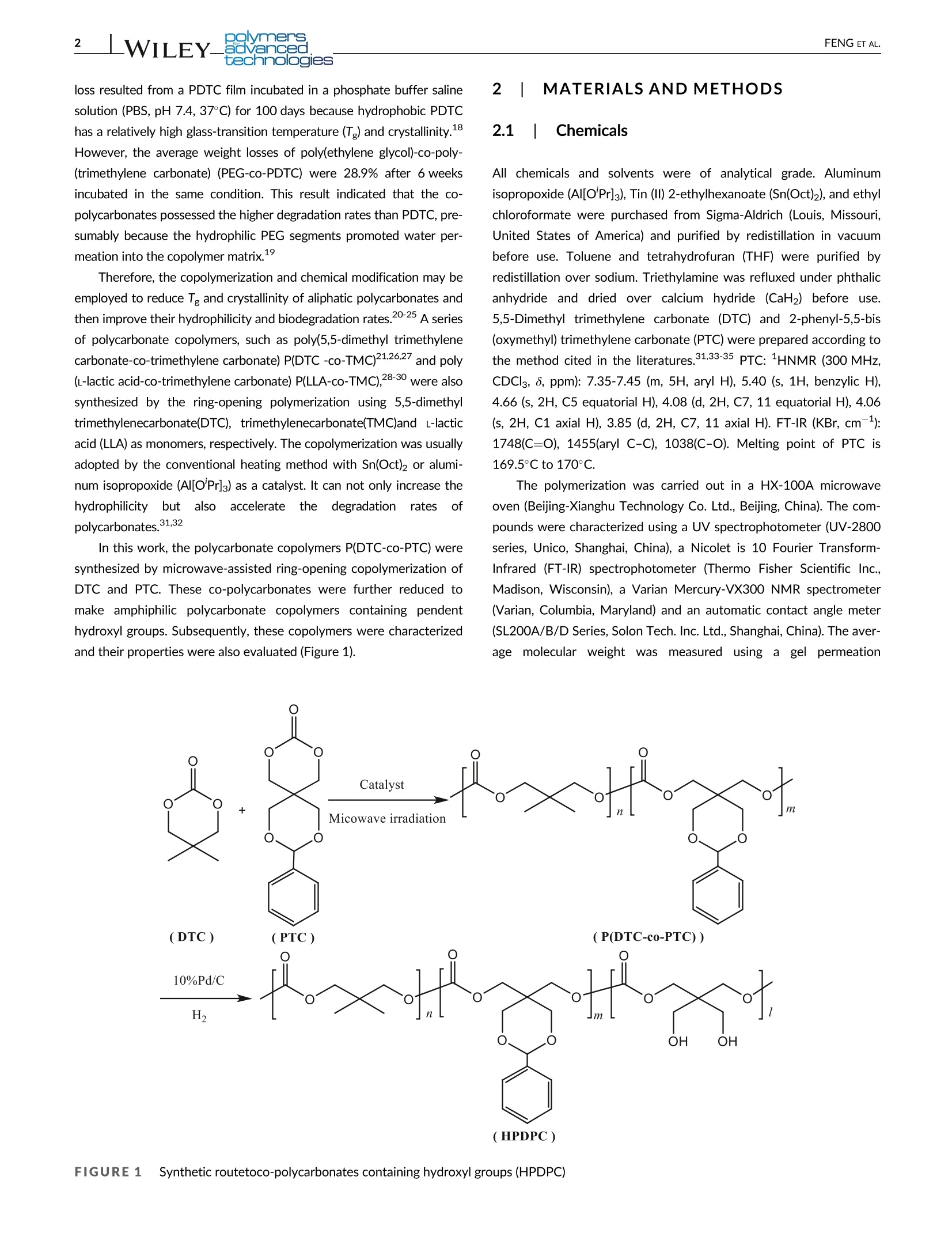
Received: 15 February 2021 Revised: 9 April 2021Accepted: 9 April 2021DOI: 10.1002/pat.5351 nensadvancedtechnologies2_WILEY-FENG ET AL. RESEARCHARTICLE Bilymens旧nce WILEYtechnologies Microwave-assisted ring-opening copolymerization andproperty of polycarbonates Tian-Ji Feng Li-Li Mei Fan Liu D Guo-Ping Yan Ye Yuan Qing-Zhong Guo School of Materials Science and Engineering,Wuhan Institute of Technology, Wuhan, China CorrespondenceFan Liu and Guo-Ping Yan, School of MaterialsScience and Engineering, Wuhan Institute ofTechnology, Wuhan 430205, China.Email: 5245934@163.com(F.L.) andguopyan2006@163.com (G.-P. Y.) Funding information Innovative Talents Project of Yellow CraneTalents Plan of Wuhan City, Grant/AwardNumber: [2017] No.1; National Key R&DProgram of China, Grant/Award Numbers:2018YFB1105502,2016YFB1101302; Pre-funded Science and Technology Projects ofWuhan, Grant/Award Number:2020020601012252;Scientific Research FundProject of Wuhan Institute of Technology,Grant/Award Number: K201861; Scientificresearch projects for high level talents in thenew century, Grant/Award Number: [2017]No.344; South Hubei Talents Project ofInnovation and Entrepreneurship, Grant/Award Number: [2019] No.11; WuhanInstitute of Technology Abstract Recently, the microwave-assisted heating method has become a commonly used andenvironmentally friendly heating technology in the field of organic and polymeric syn-thetic chemistry. A series of poly(5,5-dimethyl trimethylenecarbonate-co-2-phenyl-5,5-bis[oxymethyl] trimethylenecarbonate) (P[DTC-CO-PTC]) were synthesized bymicrowave-assisted ring-opening polymerization of 5,5-dimethyl trimethylene car-bonate (DTC) and 2-phenyl-5,5-bis(oxymethyl) trimethylene carbonate (PTC) usingtin(II) 2-ethylhexanoate and aluminum isopropoxidase, the catalysts. These co-polycarbonates were further reduced by a palladium-carbon catalyst (Pd/C catalyst,10%) to make partly deprotectedpolycarbonates (HPDPC). These two co-polycarbonates were characterized by gel permeation chromatography,HNMR,Fourier transform infrared spectroscopy, UV, differential scanning calorimetry, andautomatic contact-angle measurements. The influences of the microwave irradiationtime, microwave power, monomer feed molar ratio, different catalysts, and mono-mer/catalyst feed molar ratio on the molecular weights of co-polycarbonates werealso investigated. In vitro water absorption, degradation and drug release tests indi-cated that partly deprotected co-polycarbonate HPDPC possessed greater hydrophi-licity, faster degradation rate, and faster drug release rate than that of correspondingP(DTC-CO-PTC). Therefore, microwave-assisted polymerization is a clean and cheapheating method and can be used for ring-opening copolymerization of carbonates,which enhancesthe hydrophilicityandbiodegradationrate ofaliphaticpolycarbonates. KEYWORDS biodegradable polycarbonates, biodegradability, drug controlled release, microwave-assisted 1 INTRODUCTION Recently, the microwave irradiation has become an environmentalfriendly heating and green technology in the field of organic and poly-meric synthetic chemistry.Compared with the conventional heatingmethod, the microwave-assisted polymerization possesses the uniqueadvantages, such as high molecular weight,high yield and short reac-tion time, and controllable and rapid bulk heating capacity. As a cleanand cheap heating method,microwave-assisted polymerization hasbeen used for the ring-opening polymerization of carbonate and caprolactone, polymerization of vinyl monomers, and condensationpolymerization for esters,amides, imides, and urethanes’as well asthe curing of epoxy.10,11 Biodegradable aliphatic polycarbonates,such as poly(5,5-dimethyl trimethylenecarbonate) (PDTC)13 and poly(tri-methylenecarbonate) (PTMC),14 display low toxicity, good biodegrada-tion, biocompatibility, and elasticity and then have been widelyattracted to the application in biomedical fields, such as drug deliveryand tissue engineering.13-17 The biodegradation rate of aliphatic poly-carbonate mostly depends on their structures. Less than 3% weight loss resulted from a PDTC film incubated in a phosphate buffer salinesolution (PBS, pH 7.4, 37°C) for 100 days because hydrophobic PDTChas a relatively high glass-transition temperature (T ) and crystallinity.However, the average weight losses of poly(ethylene glycol)-co-poly-(trimethylene carbonate) (PEG-co-PDTC) were 28.9% after 6 weeksincubated in the same condition. This result indicated that the co-polycarbonates possessed the higher degradation rates than PDTC, pre-sumably because the hydrophilic PEG segments promoted water per-meation into the copolymer matrix. Therefore, the copolymerization and chemical modification may beemployed to reduce Tg and crystallinity of aliphatic polycarbonates andthen improve their hydrophilicity and biodegradation rates.20-25 A seriesof polycarbonate copolymers, such as poly(5,5-dimethyl trimethylenecarbonate-co-trimethylene carbonate) P(DTC-Co-TMC)21,26,27 and poly(L-lactic acid-co-trimethylene carbonate) P(LLA-co-TMC),28-30 were alsosynthesized by the ring-opening polymerization using 5,5-dimethyltrimethylenecarbonate(DTC), trimethylenecarbonate(TMC)and L-lacticacid (LLA) as monomers, respectively. The copolymerization was usuallyadopted by the conventional heating method with Sn(Oct)2 or alumi-num isopropoxide (Al[O'Pr]3) as a catalyst. It can not only increase thehydrophilicity but alsoaccelerate the degradationrates ofpolycarbonates.31,32 In this work, the polycarbonate copolymers P(DTC-co-PTC) weresynthesized by microwave-assisted ring-opening copolymerization ofDTC and PTC. These co-polycarbonates were further reduced tomake amphiphilic polycarbonate copolymers containing pendenthydroxyl groups. Subsequently, these copolymers were characterizedand their properties were also evaluated (Figure 1). 2 MATERIALS AND METHODS 2.1 Chemicals All chemicals and solvents were of analytical grade. Aluminumisopropoxide (AI[O'Pr]3), Tin (II) 2-ethylhexanoate (Sn(Oct)2), and ethylchloroformate were purchased from Sigma-Aldrich (Louis, Missouri,United States of America) and purified by redistillation in vacuumbefore use. Toluene and tetrahydrofuran (THF) were purified byredistillation over sodium. Triethylamine was refluxed under phthalicanhydride and dried over calcium hydride (CaH2) before use.5,5-Dimethyl trimethylene carbonate (DTC) and 2-phenyl-5,5-bis(oxymethyl) trimethylene carbonate (PTC) were prepared according tothe method cited in the literatures.31,33-35 PTC: 1HNMR (300 MHz,CDCl3, S, ppm): 7.35-7.45 (m, 5H, aryl H), 5.40 (s, 1H, benzylic H),4.66 (s,2H, C5 equatorial H), 4.08 (d, 2H, C7, 11 equatorial H), 4.06(s, 2H, C1 axial H), 3.85 (d, 2H, C7, 11 axial H). FT-IR (KBr, cm-1):1748(C=O), 1455(aryl C-C), 1038(C-O). Melting point of PTC is169.5°C to 170°C. The polymerization was carried out in a HX-100A microwaveoven (Beijing-Xianghu Technology Co. Ltd., Beijing, China). The com-pounds were characterized using a UV spectrophotometer (UV-2800series, Unico, Shanghai, China), a Nicolet is 10 Fourier Transform-Infrared (FT-IR) spectrophotometer (Thermo Fisher Scientific Inc.,Madison, Wisconsin), a Varian Mercury-VX300 NMR spectrometer(Varian, Columbia, Maryland) and an automatic contact angle meter(SL200A/B/D Series, Solon Tech. Inc. Ltd., Shanghai, China).The aver-age molecular weight was measured usinggaa gel permeation (HPDPC) chromatography (GPC) with a Waters 2965D separations module, aWaters 2414 refractive-index detector, Shodex K802.5 and K805 col-umns with a Shodex K-G guard column, a polystyrene standard, anddimethylformamide (DMF) as a solvent (flow rate was 1.0 mL/min;column temperature was 323 K; detector temperature was 323 K)(Waters, Milford, Maryland). 2.2 Microwave-assisted ring-openingcopolymerization of DTC and PTC DTC (0.52 g, 4.0 mmol, 1 equiv.) and PTC (1.0 g, 4.0 mmol, 1 equiv.)were added to a polymerization tube and then dried by several cyclesof argon purging followed by exposure to high vacuum. A solution ofSn(Oct)2 in dry toluene (0.1 mol/L, 80 pL,1/1000 equiv.) was added tothe dried mixture via a syringe. After further drying under high vac-uum, the tube was sealed and subjected to microwave irradiation at apredetermined power for a period of time (5-50 minutes). The powerwas recorded once a second. The resultant solid residue was dissolvedin dichloromethane (2 mL) and then reprecipitated using ethanol anddiethyl ether (1:1 v/v) (40 mL). Subsequently, the precipitated solidwas filtered, washed by ethanol and diethyl ether (1:1 v/v), and driedin vacuo for 48 hours to yield white powders of poly(5,5-dimethyltrimethylene carbonate-co-2-phenyl-5,5-bis(oxymethyl) trimethylenecarbonate) (P(DTC-CO-PTC)) (1.25 g, 82%).1HNMR (CDCl3, 8, ppm):7.35-7.5 (m,CH5-), 5.50 (s,C。H5-CH-[O]2-),4.58 (s,-COO-CH2C[CH2O-]2-CH2O-), 4.15 (t, -COO-CH2C[CH2O-]2-CH2O-), 4.05(s,-COO-CH2C[CH2O-]2-CH2O-), 3.98(s,-C[CH3]2-CH2-O-), 1.01(s,CH3); IR (KBr, cm-1): 2940, 2864 (-CH2), 1752(C=O), 1473(arylC-C), 1386(C-C), 1100(C-O); UV (CHCl3, A, nm):228,256. The co-polycarbonates P(DTC-co-PTC) with different average compositionsof DTC and PTC repeat units (mol%) were synthesized using the samemethod under the different reaction conditions. The number-averagemolecular weight (M) was measured by GPC, and the average compo-sitions of DTC and PTC repeat units (mol%) were determinedby1HNMR. 2.3 Deprotection of P(DTC-Co-PTC) A Pd/C catalyst (10%, 0.1 g) and 30mL of anhydrous methanol wereboth added to a solution of P(DTC-co-PTC) (1.0 g, 0.0026 mol) dis-solved in DMF (30 mL). Hydrogen was continually inflated into thereaction mixture for 48 hours at 60°C under atmospheric pressure.The mixture was filtered and the catalyst was then removed. Afterevaporation under reduced pressure, the residue was precipitatedusing n-hexane. The precipitated solid was filtered, washed by n-hexane,and dried in vacuo for 48 hours to yield a light yellow powders ofpartly deprotected co-polycarbonate: poly(5,5-dimethyl trimethylenecarbonate-co-5,5-bis(hydroxylmethyl) trimethylene carbonate-co-2-phenyl-5,5-bis(oxymethyl)trimethylene carbonate) (HPDPC, 0.78 g,78%).1HNMR (CDCl3, 8, ppm): 7.35-7.5(m, C。H5-), 5.40 (s, CHs-CH-[O]2-), 4.68 (s, -COO-CH2C[CH2O-]2-CH2O-), 4.59(ss,, HOCH2C[CH2O]2-CH2OH),4.15(s, -COO-CH2C[CH2O-]2- CH2O-), 4.05 (s,-COO-CH2C[CH2O-]2- CH2O-), 3.98 (s, -C[CH3]2-CH2-O-), 1.01(s,CH3); IR(KBr, cm-1): 3300 (-OH), 2958, 2858 (-CH2), 1750 (C=O), 1475, 1378 (C-C), 1110 (C-O); UV (CHCl3, a,nm): 228,256. The molecular weight (M ) was measured by GPC andthe average compositions of repeat units (mol%) including DTC,PTC, and deprotected PTC containing hydroxyl groups were deter-mined by 1HNMR. 2.4 In vitro degradation test The co-polycarbonates (0.1 g) were pressed into tablets and thendried in vacuum for 24 hours. The tablets were suspended in 10 mLof PBS in a dialysis bag. The dialysis bag was sealed and then slowlyshaken in 90 mL of PBS (pH 7.4) at 37°C in a 250 mL Erlenmeyerflask. At predetermined time intervals, the samples were taken out ofthe degradation medium, rinsed with distilled water, and then dried invacuo for 48 hours. The water absorption and weight loss werecalculated. 2.5 hIn vitro drug-release study Fluorouracil (5-Fu, 10 mg) as a model drug and P(DTC-CO-PTC) orpartly deprotected copolymers HPDPC (100 mg) were dissolved in20 mL of THF. The solution was homogenized by sonication for30 seconds and then allowed to evaporate. The resulting film was col-lected and pressed in a tablet press to produce 5-Fu-incorporatedP(DTC-co-PTC) tablets andd5-Fu-incorporated HPDPC tablets,respectively. The tablets were suspended in 10 mL of PBS in a dialysis bag.The dialysis bag was sealed and then slowly shaken in 90 mL of PBSat 37°C in a 250 mL Erlenmeyer flask. Aliquots of the solution outsidethe dialysis membrane (25mL) were replaced with 25 mL of PBS atvarious times intervals and tested at 256 nm by a high-performanceliquid chromatography spectrophotometer (HPLC). The changes ofthe concentrations of 5-Fu were obtained from curves of the absorp-tion A versus concentration C of 5-Fu in PBS based on Lambert-Beer law. 2.6 Statistical analysis All results were expressed as mean differences and were tested forsignificance by a t-test, P<0.05 being considered a significantdifference. 3 RESULTS AND DISCUSSION 3.1 Synthesis of co-polycarbonates The co-polycarbonates P(DTC-Co-PTC) were synthesized bymicrowave-assisted ring-opening copolymerization of DTC and PTC using Al(O'Pr)s Feed Molar Ratio (mol/mol) M Mw/M, Tg(C) M Mw/MC Tg(C) 1:8 12,370 1.11 41.6 11,400 1.12 39.1 1:4 14,380 1.17 42.6 12,910 1.11 41.6 1:2 18,300 1.14 45.2 17,230 1.15 44.2 1:1 26,060 1.24 47.9 18,490 1.21 46.7 2:1 16,300 1.15 44.5 15,600 1.17 43.8 4:1 13,100 1.14 42.1 15,190 1.33 42.6 8:1 12,970 1.15 41.4 12,260 1.15 40.3 Microwave power=320 W, irradiation time= 12 minutes,[M]/[C]=1000:1.Catalyst=Al(O'Pr)3. TABLE 1 Effect of monomerDTC/PTC feed molar ratio oncopolymerization when microwavepower was 320 W and Sn(Oct)2 as the catalysts. The FT-IR spectra of co-polycarbonatesshowed a characteristic peak in 1752 cmwhich represents theabsorption peak of C=O groups. In the HNMR spectra of co-poly-carbonates, the typical signals for co-polycarbonate structures can beobserved at 1.01 ppm (DTC repeat units: -CH3) and 7.35-7.5 ppm(PTC repeat units:-C6H5),respectively. Thus, the average compositionsof DTC and PTC repeat units (mol%) in co-polycarbonate structure canbe calculated according to the integration values of the -CH3 and-CHs peaks. The effects of polymerization conditions including themicrowave irradiation time, microwave power, molar ratio of mono-mers, and monomer/catalyst molar ratio on the molecular weights ofco-polycarbonates are listed in Tables 1 to 5. The effects of monomer DTC/PTC feed molar ratio on the poly-merization with Al(O'Pr)s and Sn(Oct)2 as catalysts are shown inTables 1 and 2. The polymerization condition was listed as follows:the monomer/catalyst feed molar ratio [M]/[C] was 1000/1, micro-wave powers were 320 and 160 W, and microwave irradiation timewas 12 minutes. The molecular weight, glass transition temperature,and water contact angle of P(DTC-co-PTC) increased along with theincrease of monomer DTC/PTC feed molar ratio from 1:8 to 1:1andthen decreased when monomer DTC/PTC feed molar ratio continu-ously increased from 1:1 to 8:1. The highest Mn values ofco-polycarbonates were 18,490 and 26,060 and glass transition tem-peratures were 46.7°C and 47.9°C, respectively, when the monomerDTC/PTC feed molar ratio was 1:1 and microwave power was 320 Wusing Sn(Oct)2 and Al(O'Pr)3 as the catalysts (Table 1). Meanwhile, thehighest Mn values of co-polycarbonates were 17,040 and 16,710,respectively (Table 2) when the monomer DTC/PTC feed molar ratiowas 1:1 and microwave power was 160 W using Sn(Oct)2 andAl(O'Pr)s as the catalysts. The influence of the monomer/catalyst feed molar ratio [M]/[C],microwave power, and microwave irradiation time on the copolymeri-zation are shown in Tables 3 and 4.The polymerization condition waslisted as follows: the monomer DTC/PTC feed molar ratio was 1/1,monomer/catalyst feed molar ratio [M]/[C]varied from 250/1 to2000/1, microwave powers were 320 and 160 W, and microwaveirradiation time was changed from 5 to 50 minutes. The molecularweight of P(DTC-CO-PTC) increased in pace with the increase of TABLE2 Effect of monomer DTC/PTC feed molar ratio oncopolymerization when microwave power was 160 W Molar Ratio (mol/mol) M Mw/Mn' M Mw/M 1:8 6390 1.38 7490 1.43 1:4 9510 1.20 9690 1.11 1:2 14,660 1.23 16,790 1.17 1:1 16,710 1.17 17,040 1.15 2:1 13,360 1.16 15,420 1.10 4:1 11,470 1.16 11,690 1.20 8:1 9600 1.21 11,590 1.30 Microwave power= 160 W, irradiation time=20 minutes,[M]/[C]=1000:1.Catalyst=Al(O'Pr)3.Catalyst= Sn(Oct)2. monomer/catalyst feed molar ratio [M]/[C] from 250/1 to 1000/1and then decreased when the monomer/catalyst feed molar ratio[M]/[C] increased from 1000/1 to 2000/1. The highest Mvalues ofco-polycarbonates were 18,490 and 18,300 when the monomerDTC/PTC feed molar ratio was 1:1 using Sn(Oct)2 and Al(O'Pr)3 as thecatalysts, respectively, the monomer/catalyst feed molar ratio[M]/[C] was 1000/1, microwave power was 320 W, and microwaveirradiation time was 12 minutes. The highest Mn value of the co-polycarbonate was 18,500 when the monomer DTC/PTC feed molarratio was 1:1 using Sn(Oct)2 as a catalyst, monomer/catalyst feedmolar ratio [M]/[C] was 1000/1, microwave power was 160 W, andmicrowave irradiation time was 30 minutes. These results indicatedthat the overly high dosage of catalysts resulted in the reduction ofthe number average molecular weight of co-polycarbonates. The effects of microwave power and irradiation time on the copo-lymerization are shown in Table 4. The polymerization condition waslisted as follows: the monomer DTC/PTC feed molar ratio was 1/1,monomer/catalyst feed molar ratio [M]/[C] was 1000/1, microwavepowers were 320 and 160 W, and microwave irradiation times variedfrom 5 to 50 minutes. The number average molecular weights ofP(DTC-CO-PTC) increased in the wake of the increase of microwave Catalyst Microwave Power (W) Time (min) [M]/[C] Mn M/Mn Sn(Oct)2 320 12 2000 17,310 1.14 Sn(Oct)2 320 12 1000 18,490 1.21 Sn(Oct)2 320 12 500 18,460 1.13 Sn(Oct)2 320 12 250 15,240 1.08 Al(O'Pr)3 320 12 2000 17,400 1.16 Al(O'Pr)3 320 12 1000 18,300 1.14 Al(O'Pr)3 320 12 500 12,150 1.05 Al(O'Pr)3 320 12 250 11,190 1.07 Sn(Oct)2 160 30 2000 18,300 1.23 Sn(Oct)2 160 30 1000 18,500 1.18 Sn(Oct)2 160 30 500 16,330 1.16 Sn(Oct)2 160 30 250 12,370 1.51 Monomer DTC/PTC feed molar ratio (mol/mol)=1:1. TABLE4 Effect of microwave power and irradiation time oncopolymerization Microwave Power (W) Time (min) M Mw/M, 160 5 5730 1.5 160 10 10,870 1.39 160 12 12,870 1.16 160 15 13,320 1.2 160 20 17,040 1.15 160 25 17,000 1.16 160 30 18,500 1.19 160 40 5870 1.45 160 45 5760 1.46 160 50 4020 1.06 320 5 11,350 1.12 320 10 11,500 1.07 320 12 18,490 1.21 320 15 17,850 1.06 320 20 11,280 1.33 320 30 9320 1.13 Catalyst= Sn(Oct)2, [M]/[C]=1000:1,monomerDTC/PTC feed molarratio (mol/mol)=1:1. irradiation time from 5 to 30 minutes and subsequently decreasedwhen the microwave irradiation time increaseddfrom30 to50 minutes, while the microwave power was 160 W. The highest Mnvalue of the co-polycarbonate was 18,500 when the monomerDTC/PTC feed molar ratio was 1:1 using Sn(Oct)2 as a catalyst, micro-wave power was 160 W, and microwave irradiation time was30 minutes. When the copolymerization condition was changed tomicrowave power of 320 W using Sn(Oct)2 as a catalyst, the changeof Mn value was similar to the earlier experimental results. The num-ber average molecular weights of P(DTC-CO-PTC) increased when themicrowave irradiation time increased from 5 to 12 minutes and thendecreased when the microwave irradiation time increased from 12 to30 minutes, while the microwave power was 320 W. The highest Mn value of co-polycarbonate was 18,490 when the monomer DTC/PTCfeed molar ratio was 1:1 using Sn(Oct)2 as a catalyst, microwavepower was 320 W, and microwave irradiation time was 12 minutes.The decrease of Mn at the prolonged microwave irradiation time canbe explained by the random thermal degradation of P(DTC-CO-PTC).The longer microwave irradiation time and higher microwave irradia-tion power probably led to the degradation and interchange esterifica-tion reaction of co-polycarbonates and induced in the decrease ofnumber average molecular weights because the thermal degradationrate was faster than the copolymerization rate under the conditions ofhighmicrowave irradiation power and long microwaveirradiation time. The Mn of P(DTC-co-PTC) reached 11,350 by microwave-assistedring-opening copolymerization when the microwave irradiation timewas only 5 minutes, monomer DTC/PTC feed molar ratio was 1/1,monomer/catalyst feed molar ratio [M]/[C] was 1000/1 usingSn(Oct)2 as a catalyst, and microwave power was 320 W. However, ittook nearly 8 hours at 180°C to produce the same Mn of P(DTC-co-PTC) with the ring-opening copolymerization using the conventionaltheating method. Although this is far from being definitely establishedthe reaction mechanism of microwave-assisted ring-opening copoly-merization, these results suggest that the microwave energy provide amore efficient synthesis method to polymerization. The reason for thisphenomenon is that polar molecules undergo directional polarizationand rotational movement under microwave conditions. Due to theinteraction and thermal movement of the molecules, the rotationalmovement of the molecules is blocked and internal friction occurs. Asa result, the temperature rises rapidly, which forms a violent reactionand increases the active of polymerization.36-38 3.2 Synthesis of partly deprotectedcopolycarbonates containing hydroxyl groups(HPDPCh P(DTC-co-PTC) was further reduced using a Pd/C catalyst to makepartly deprotected co-polycarbonates containing hydroxyl groups TABLE5 Water contact angle (WCA), surface energy,and workof adhesion of P(DTC-co-PTC)a Feed Molar Ratio Water Contact Surface Work of (mol/mol) Angle () Energy (J) Adhesion (J) 1:8 70.1 32.69 97.56 1:4 73.6 29.96 97.39 1:2 75.9 28.17 90.58 1:1 80.6 24.60 84.65 2:1 74.9 28.93 91.78 72.9 30.46 94.18 71.6 31.51 95.79 Microwave power = 320 W, irradiation time = 12 minutes,[M]/[C]=1000:1, catalyst= Al(O'Pr)3. (HPDPC). The copolymerization condition of corresponding P(DTC-co-PTC) was listed as follows: the monomer DTC/PTC feed molarratios were1:4, 1:1, and 4:1, using Al(O'Pr)3 as a catalyst with micro-wave power of 320 W and irradiation time of 12 minutes. The watercontact angle (WCA), surface energy, and work of adhesion of P(DTC-Co-PTC) are showed in Table 5. WCA of P(DTC-CO-PTC) increasedwhen the monomer DTC/PTC feed molar ratio increased from 1:8 to1:1 and then decreased when monomer DTC/PTC feed molar ratioincreased from 1:1 to 8:1. The highest WCA value was 80.6° whenthe monomer DTC/PTC feed molar ratio was 1:1. It could be con-cluded that the hydrophilicity of P(DTC-co-PTC) improved when themonomer DTC/PTC feed molar ratio increased from 1:8 to 1:1 andthen decreased when the monomer DTC/PTC feed molar ratioincreased from 1:1 to 8:1. The introduction of hydroxyl groups to partly deprotected co-polycarbonate HPDPC is expected to improve amphiphilic property,enhance biodegradation rate, increase the drug-release rate, andpotentially provide a platform for chemical modification to readilyattach drugs and tissue-or organ-targeting groups in drug deliverysystems. The FT-IR spectra of HPDPC showed a characteristic peak in3300 cm-1, which represented the absorption peak of -OH groups,moreover, the decrease of the intensity of characteristic peak at1750 cm-1 representedlthe absorption peak of C=0 groupsdecreased. In the 1HNMR spectra of HPDPC, the typical signals forbenzene rings (-CHs) can be observed at 7.35 to 7.5 ppm, and theintegration values of the peaks decreased. The 1HNMR and IR spectrashowed that the benzene ring and hydroxyl group both existed in thestructure of HPDPC, and then these results indicated that a part ofbenzene rings in PTC repeat unit of co-polycarbonates were reduced.The result demonstrated that the hydroxyl proportions of HPDPC(mol%) were 46.1%, 44.2%, and 45.8%, respectively, when the mono-mer DTC/PTC feed molar ratios in copolymerization process were1:4, 1:1, and 4:1, respectively. Compared with P(DTC-CO-PTC), WCA of partly deprotected co-polycarbonates HPDPC decreased because the hydrophilicity of co-polycarbonates was enhanced, whereas some benzene rings of repeatunit PTC segments in co-polycarbonates were replaced by hydroxyl TABLE 6 WCA, surface energy and work of adhesion of partlydeprotected co-polycarbonates containing hydroxyl groups HPDPC Feed Molar Ratio Water Contact Surface Work of (mol/mol) Angle () Energy(J) Adhesion (J) 1:4 63.3 38.24 105.52 1:1 67.9 34.47 100.18 4:1 64.0 37.83 104.96 Microwave power= 320 W, irradiation time = 12 minutes,[M]/[C]=1000:1, catalyst=Al(O'Pr)3. groups. After removal of the hydrophobic benzyl-protected group andimporting the hydrophilic group (-OH) in HPDPC chain, the hydrophi-licity of HPDPC improved and then their WCA decreased (Table 6). 3.3 In vitro degradation test Hydrolysis tests were performed to appraise the copolymeric degrad-ability. The in vitro degradation of P(DTC-co-PTC) and partly dep-rotected co-polycarbonates HPDPC were measured in PBS. P(DTC-co-PTC) was synthesized at the copolymerization condition listed asfollows: the monomer DTC/PTC feed molar ratios were 1:4, 1:1, and4:1, respectively, using Al(O'Pr)s as a catalyst with 320 W of micro-wave power and irradiation time of 12 minutes. P(DTC-co-PTC) werefurther reduced by a Pd/C catalyst to make partly deprotected co-polycarbonate HPDPC. Their degradation properties were denoted bythe water absorption (Figures 2 and 4) and weight loss (Figures 3 and5) during 240 days in PBS at 37°C. The water absorption and weightloss of HPDPC were all higher than those of the correspondingP(DTC-Co-PTC) after 240 days of degradation. The water absorptions of partly deprotected co-polycarbonatesHPDPC were 42.9%, 30.2%, and 40.1%, respectively after 100 daysof degradation (Figure 4) when the monomer DTC/PTC feed molarratio in copolymerization process were 1:4, 1:1, and 4:1, respectively.However, the water absorption of the corresponding P(DTC-CO-PTC)were 5.4%, 3.74%, and 4.5%, respectively under the same conditions(Figure 2) when the monomer DTC/PTC feed molar ratio in copoly-merization process were 1:4, 1:1, and 4:1, respectively. Therefore, thedegradation rate of HPDPC became faster than that of thecorresponding P(DTC-CO-PTC), presumably because hydroxyl groupsenhanced the hydrophilicity, promoted water absorption and perme-ation into the copolymer matrix, and then improved their biodegrada-tion rates. During the degradation process in PBS, P(DTC-co-PTC) with dif-ferent monomer DTC/PTC feed molar ratio in copolymerization expe-rienced almost same weight loss. P(DTC-CO-PTC) with monomerDTC/PTC feed molar ratio of 1:1 in copolymerization had lowermolecular weight loss and degradation rate than P(DTC-CO-PTC) withDTC/PTC feed molar ratio of 4:1 and 1:4. Meanwhile, P(DTC-Co-PTC)exhibited low weight loss after 240 days. The weight losses of P(DTC-co-PTC)were only 2.15%, 1.37%, and 1.96% after 240 days when the FIGURE 2 Water absorption of P(DTC-CO-PTC) FIGURE3 Weight loss of P(DTC-co-PTC) FIGURE 4 Water absorption of HPDPC monomer DTC/PTC feed molar ratio in copolymerization were1:4,1:1, and 4:1, respectively (Figure 3). In contrast, the weight losses ofHPDPC were 15.5%,7%, and 13.8%, respectively, when the monomerDTC/PTC feed molar ratios in copolymerization were 1:4, 1:1, and4:1, respectively (Figure 5). The results suggested that the degradationrate of HPDPC be much faster than that of P(DTC-co-PTC) and has FIGURE 5 Weight loss of HPDPC FIGURE6 Release profiles of 5-Fu from P(DTC-co-PTC) relations with the molecular weight and the hydroxyl content of co-polycarbonates. 3.4 In vitro drug-release property of copolymers The 5-Fu release properties of P(DTC-co-PTC) and partly deprotectedco-polycarbonate HPDPC are shown in Figures 6 and 7. P(DTC-co-PTC) was synthesized at the copolymerization conditions listed as fol-lows: the monomer DTC/PTC feed molar ratios were 1:4, 1:1, and4:1, respectively, using Al(O'Pr)s as a catalyst with microwave powerof 320 W and irradiation time of 12 minutes. P(DTC-Co-PTC) wasfurther reduced by a Pd/C catalyst to produce partly deprotected co-polycarbonates HPDPC. The substantial releases of the 5-Fu-incorporated P(DTC-Co-PTC) tablets and 5-Fu-incorporated HPDPCtablets were maintained for 25 days of measurement. The 5-Fu-incorporated P(DTC-CO-PTC) tablets and 5-Fu-incorporated HPDPCtablets displayed steady drug-release rates and good drug controlledrelease properties. Compared with 5-Fu-incorporated PDTC tablets,the 5-Fu-incorporated P(DTC-co-PTC) tablets possessed the faster drug-release rates, presumably due to the high degradation rate and drug FIGURE 7 Release profiles of 5-Fu from partly deprotected co-polycarbonates HPDPC diffusion coefficient of P(DTC-co-PTC). Moreover, the 5-Fu releaserate changed when the monomer DTC/PTC feed molar ratiodecreased from 4:1 to 1:4. The cumulative percentage release ofP(DTC-co-PTC) (with monomerDTC/PTC molar ratio: 4:1, 1:1, and 1:4in the copolymerization process) reached 15.80%, 11.58%, and36.31%, respectively, after 36 days of drug-release (Figure 6). The partly deprotected co-polycarbonates HPDPC had fasterdrug-release rates than that of the corresponding P(DTC-CO-PTC).The cumulative percentage release of HPDPC (with monomerDTC/PTC feed molar ratio:4:1, 1:1, and 1:4) were 27.50%,19.19%,and 43.12%, respectively, after 25 days of drug-release (Figure 7). Thisresult indicated that hydroxyl groups enhanced the hydrophilicity,water absorption, biodegradation rates, and drug diffusion coefficientsof co-polycarbonates. 4 l CCONCLUSIONS Theco-polycarbonatesP(DTC-co-PTC))were synthesized bymicrowave-assisted ring-opening polymerization of DTC and PTCusing Sn(Oct)2 and Al(O'Pr)s as the catalysts, respectively. P(DTC-co-PTC) was further reduced using a Pd/C catalyst to produce partly dep-rotected co-polycarbonates HPDPC. HPDPC displayed faster degra-dation rate, faster drug-release rate, and more hydrophilicity than thatof corresponding P(DTC-CO-PTC). All co-polycarbonates possessedsteady drug-release rates and good controlled release properties. ACKNOWLEDGMENTS This work was supported by the National Key R&D Program of China(Grant No. 2018YFB1105502,2016YFB1101302), Frontier Project ofApplication Foundation of Wuhan Former Funded Science and Technol-ogy Program (Grant No. 2020020601012252), Innovative Talents Projectof Yellow Crane Talents Plan of Wuhan City ([2017] No.1), South HubeiTalents Project of Innovation and Entrepreneurship ([2019] No.11), Scien-tific research projects for high level talents in the new century ([2017]No.344), and Scientific Research Fund Project of Wuhan Institute ofTechnology (Grant No. K201861), Hubei Province, China. The authors declare no conflicts of interest. ORCID FanLiuu https://orcid.org/0000-0002-2865-2372 REFERENCES 1. Adam D. Microwave chemistry: out of the kitchen. Nature. 2003;421(6923):571-572. 2. Tanan W, Panpinit S, Saengsuwan S. Comparison of microwave-assisted and thermal-heated synthesis of P(HEMA-Co-AM)/PVA inter-penetrating polymer network (IPN) hydrogels for Pb(II) removal fromaqueous solution: characterization, adsorption and kinetic study. EurPolym J. 2021;143:110193. 3. MishraA, Vats T, Clark JH. Microwave assisted polymerization: Super-absorbent polymerwith improved properties. J Appl Polym Science,2016;133(16):43325. 4. BEujok S, Konefat M, Abbrent S, et al. lonic liquid-functionalized LDH ascatalytic-initiating nanoparticles for microwave-activated ring openingpolymerization of e-caprolactone. React Chem Eng. 2020;5(3):506-518. 5. Kredatusova J, Benes H, Livi S, et al. Influence of ionic liquid-modifiedLDH on microwave-assisted polymerization of e-caprolactone. Poly-mer. 2016;100:86-94. 6. Katharina Reitz A, Sun Q, Wilhelm R, Kuckling D. The use of stablecarbene-CO2 adducts for the polymerization of trimethylene carbon-ate. J Polym Sci. 2017;55(5):820-829. 7. Novanna M, Kannadasan S, Shanmugam P. Microwave assistedsynthesis and photophysical properties of blue emissive 2-amino-3-carboxamide-1,1'-biaryls and 4-(arylamino)-[1,1'-biphenyl]-3-carboxamides via Suzuki and Chan-Evans-Lam coupling. Dyes Pigm.2020;174:108015. ( 8. Sakthinathan S, Suresh R, K amalakkannan D, e t al. Microwave assisted s ynthesis, spectral correlation and antimicrobial evaluation ofsome aryl i mines. J Chil Chem Soc. 2018;63:3918-3923. ) 9. EBiswas A, Kim S, Gomez A, Buttrum M, Boddu V, Cheng HN. Micro-wave-assisted synthesis of sucrose polyurethanes and their semi-interpenetrating polymer networkswith polycaprolactonee andsoybean oil. Ind Eng Chem Res.2018;57(9):3227-3234. ( 10. Zhao X , Wang XL , Tian F, A n WL, Xu S, Wa n g YZ. A fast and mildclosed-loop recycling o f anhydride-cure d epoxy through microwave- assisted catalytic degradation by trifunctional amine and s ubsequent reuse without separation. Green Chem. 2 019;21(9):2487-2493. ) 11. Pal R, Akhtar MJ, Kar KK. Microwave-assisted curing of siliconcarbide-reinforced epoxy composites: role of dielectric properties.JOM.2018;70(7):1295-1301. ( 12. Singh S, H ussain A, A leti RR, Nlooto M, Karpoormath R. Ch a pter 8- polycarbonate nanoparticles a s a promising tool f o r anticancer thera-peutics. In: Kesharwani P , P aknikar KM, Gajbhiye V, ed s . PolymericNanoparticles as a Promising Tool f or A nti-cancer T herapeutics. Lake C ity UT, USA: Academic Press; 2019:147-166. ) 13. Socka M, Michalski A, Pelin IM, et al. Preparation of biomimetic com-posites ofhydroxyapatite andstar-shaped poly(2,2-dimethyltrimethylene carbonate)s terminated with carboxyl end-groups. Poly-mer. 2020;186:122078. 14. He J, Zhang Y, Liu X, et al. Load-bearing PTMC-beta tri-calcium phos-phate and dexamethasone biphasic composite microsphere scaffoldsfor bone tissue engineering. Mater Lett. 2020;260:126939. ( 15. Hu B , Ke X J , Yan GP, e t al. Preparation and properties of polycarbon- ate microspheres containing t e tanus toxoid vaccine. J Appl Polym Sci. 2014;131(5):40084. ) ( 16. L iang J, Grijpma DW, P oot AA. Tough and b i ocompatible hybrid net- works prepared f r om methacrylated poly(trimethylene car b onate) (PTMC) and methacrylated gelatin. Eur Polym J.2020;123:109420. ) 17. Wang Y, Xi L, Zhang B, et al. Bioresorbable hydrogels prepared byphoto-initiated crosslinking of diacrylated PTMC-PEG-PTMC triblockcopolymers as potential carrier of antitumor drugs. Saudi Pharm .2020;28(3):290-299. 18. Parzuchowski PG, Jaroch M, Tryznowski M, Rokicki G. Synthesis ofnew glycerol-based hyperbranched polycarbonates. Macromolecules.2008;41(11):3859-3865. 19. Hu X, Chen X, Xie Z, Cheng H, Jing X. Aliphatic poly(ester-carbonate)s bearing amino groups and its RGD peptide grafting. J Polym Sci Pt APolym Chem. 2008;46(21):7022-7032. 20. Geng Y, He H, Liu H, Jing H. Preparation of polycarbonate/poly(lacticacid) with improved printability and processability for fused deposi-tion modeling. Polym Adv Technol. 2020;31:2848-2862. 21. Li ZM, Yan GP, Ai CW, et al. Synthesis and properties of polycarbon-ate copolymers of trimethylene carbonate and 2-phenyl-5,5-bis(hydroxymethyl) trimethylene carbonate. J Appl Polym Sci. 2012;124:3704-3713. 22. Yan GP, Li Z, Xu W, et al. Porphyrin-containing polyaspartamide gad-olinium complexes as potential magnetic resonance imaging contrastagents. Int J Pharm. 2011;407:119-125. 23. SShah TV, Vasava DV. A glimpse of biodegradable polymers and theirbiomedical applications e-polymers. 2019;19(1):385-410. 24. Ramaraju H, Akman RE, Safranski DL, Hollister SJ. Designing biode-gradable shape memory polymers for tissue repair. Adv Func Mater.2020;30(44):2002014. ( 25. S S amantaray P K , Li t tle A , H a ddleton DM, et a l . Poly ( glycolic aci d ) ( PGA): a versatile building block expanding high p e rformance and sus- t ainable bioplastic applications. Gre e n Chem. 2020;22(13):4055-4081. ) ( 26. H ou Z, Li P , G u o J, Wang J, H u J, Y ang L. The effect of m ol e cular weight on t hermal p roperties and degradation behavior of copoly-mers based on TMC a n d D T C. Polym Degrad Stab. 2020;175:109128. ) 27. Hou Z, Zhang W, Guo J, Chen Z, Hu J, Yang L. The in vitroenzymatic degradation of poly(trimethylene carbonate-co-2, 2'-dimethyltrimethylene carbonate).Eur Polym J. 2019;112:51-59. 28. Xi L, Wang Y, Su F, Zhu Q, Li S. Biocompatibility and degradationstudies of poly(L-lactide-co-trimethylene carbonate) copolymers ascardiac occluders. Materialia. 2019;7:100414. ( 29. C C ork J, Whittaker AK, C ooper-White JJ , Grondahl L. T e nsile proper- t ies and i n vitro degradation of P(TMC-CO-LLA) elastomers. J Mater Chem B. 2015;3(21):4406-4416. ) 30. Cork J, Grondah1 L, Cooper White J, et al. Electrospinning andmechanical properties of P(TMC-Co-LLA) elastomers. JMater Chem B.2017;5(12):2263-2272. 31. Zhi LL, Jian-Min Z, Zhuo RX. Synthesis and characterization of func-tional polycarbonates. Chem J Chin Univ. 2003;24(09):1730-1732. 32. Li X, Zhang Q, Zhou X, et al. Microwave-assisted ring-opening poly-merization of cyclic carbonates. J Wuhan Inst Technol. 2011;33(07):25-28. 33. Liu F, Mei LL, Tan ZL, et al. Studies on microwave-assisted ring-openingpolymerization)naand propertyy Coffpoly(9-phenyl-2,-4,8,10-tetraoxaspiro-[5,5]undcane-3-one). Chin J Polym Sci. 2016;34(11):1330-1338. 34. Mei LL, Yan GP, Yu XH, Cheng SX, Wu JY. Ring-opening copolymeri-zation and properties of polycarbonate copolymers. J Appl Polym Sci.2010;108(1):93-98. 35. Chen H, Yan GP, Li L,Ai CW, Yu XH. Synthesis, characterization, andproperties of epsilon-caprolactone and carbonate copolymers. J ApplPolym Sci. 2009;114:3087-3096. 36. Wang XL, Chen YY, Wang YZ. Synthesis of poly(p-dioxanone) cata-lyzed by Zn L-lactate under microwave irradiation and its applicationin ibuprofen delivery. J Biomat Sci Polym E. 2010;21(6):927-936. 37. Pandey A, Pranesh BA. Microwave synthesis of poly(L-lactic acid).J Biomat Sci Polym E. 2009;20(1):33-48. 38. Luef KP, Hoogenboom R, Schubert US, Wiesbrock F. Microwave-assisted cationic ring-opening polymerization of 2-oxazolines. AdvPolym Sci. 2015;274:183-208. 39. Albertsson AC, Eklund M. Influence of molecular structure on thedegradation mechanism of degradable polymers: in vitro degradationof poly(trimethylene carbonate), poly(trimethylene carbonate-co-cap-rolactone), and poly(adipic anhydride). J Appl Polym Sci. 1995;57:87-103. ( How to cite this article: Feng T-J, M ei L-L, Liu F, Yan G-P, ) ( Yuan Y, Guo Q-Z. Microwave-assisted ring-opening ) Polym Adv Technol. wileyonlinelibrary.com/journal/patC John Wiley & Sons Ltd. FIGURE Synthetic routetoco-polycarbonates containing hydroxyl groups (HPDPC) NO.101前言近来,在有机和高分子合成化学领域,微波辅助加热方法已成为一种常用的环境友好型加热技术。一系列的聚(5,5-二甲基三甲基碳酸酯-co-2-phenyl-5,5-bis[oxymethyl] trimethylenecarbonate)(P[DTC-co-PTC])是通过微波辅助开环合成的。微波辅助开环聚合5,5-二甲基碳酸三甲酯(DTC)和2-苯基-5,5-双(氧甲基)碳酸三甲酯(PTC),使用2-乙基己酸锡(II)和异丙氧基铝的催化剂。这些共混碳酸盐在钯碳催化剂(Pd/C催化剂。10%)来制造部分脱保护的聚碳酸酯(HPDPC)。这两种共混碳酸盐通过凝胶渗透色谱法、1HNMR,傅里叶变换红外光谱、紫外线、差示扫描量热法和自动接触角测量。微波辐照的影响微波照射时间、微波功率、单体进料摩尔比、不同的催化剂以及单体/催化剂进料摩尔比对共聚碳酸酯分子量的影响也被研究。体外吸水、降解和药物释放试验表明,部分脱保护的共聚碳酸酯HPDPC具有更大的亲水性、更快的降解率和更快的药物释放率,而不是相应的P(DTC-co-PTC)。因此,微波辅助聚合是一种清洁和廉价的的加热方法,可用于碳酸盐的开环共聚。它能提高脂肪族聚碳酸酯的亲水性和生物降解率。NO.202简介:最近,微波辐照已经成为有机和高分子合成化学领域的一种环境友好的加热和绿色技术。与传统的加热方法相比,微波辅助聚合具有独特的优势,如高分子量、高产率和短的反应时间,以及可控和快速的批量加热能力作为一种清洁和廉价的加热方法,3 微波辅助聚合已被用于碳酸盐和己内酯的开环聚合,4乙烯基单体的聚合,5以及酯类的缩合酯、6 酰胺、7 酰亚胺、8 和聚氨酯9 的缩合聚合,以及环氧树脂的固化。10,11 环氧树脂的固化。可生物降解的脂肪族聚碳酸酯12,如聚(5,5-)二甲基三甲碳酸盐。(PDTC)13和聚(三甲基碳酸酯(PTMC)14,显示出低毒性、良好的生物降解性、生物相容性和弹性,然后被广泛吸引到生物医学领域的应用,如药物输送和组织工程等方面被广泛吸引。13-17 脂肪族聚碳酸酯的生物降解率主要取决于其结构。在磷酸盐缓冲盐水中培养的PDTC薄膜的重量损失不到3%。溶液(PBS,pH7.4,37C)中培养100天,因为疏水性的PDTC具有相对较高的玻璃转化温度(Tg)和结晶度。然而,聚(乙二醇)-共-聚(三甲基)碳酸酯(PDTC)的平均重量损失。(三甲基碳酸酯)(PEG-co-PDTC)的平均重量损失为28.9%。在相同的条件下培养,平均重量损失为28.9%。这一结果表明,共聚碳酸盐拥有比PDTC更高的降解率,可能是因为亲水的PEG段促进了水向共聚物基体的渗透。因此,可以采用共聚和化学改性来降低脂肪族聚碳酸酯的Tg和结晶度,然后提高其亲水性和稳定性。然后改善其亲水性和生物降解率。一系列聚碳酸酯共聚物,如聚(5,5-二甲基三亚甲基碳酸酯)P(DTC-co-TMC)21,26,27和聚(L-乳酸-co-TMC)。(L-lactic acid-co-trimethylene carbonate) P(LLA-co-TMC),28-30。烯酸-co-TMC,28-30也是通过开环聚合,使用5,5-二甲基三羟甲基碳酸酯(DTC)、三羟甲基碳酸酯(TMC)和L-乳酸(LLA)作为单体,通过开环聚合合成了P(LLA-co-TMC)。分别作为单体。共聚通常采用传统的加热方法,用Sn采用传统的加热方法,以Sn(Oct)2或异丙醇铝(Al[OiPr]3)为催化剂。它不仅可以增加亲水性,还可以加速聚碳酸酯的降解速度。31,32在这项工作中,聚碳酸酯共聚物P(DTC-co-PTC)是通过微波辅助合成的开环共聚,合成了聚碳酸酯共聚物P(DTC-co-PTC)。这些共聚碳酸盐被进一步还原为制成含有垂悬羟基的两亲性聚碳酸酯共聚物。随后,对这些共聚物进行了评估(图1)祥鹄仪器在文献中的使用过程聚合是在XH-100A微波炉中进行的。(Beijing-Xianghu Technology Co. Ltd., Beijing, China)。使用紫外分光光度计(UV-2800系列,中国上海,Unico)对化合物进行表征。Nicoletis10FourierTransformInfrared(FT-IR)分光光度计(ThermoFisherScientificInc,麦迪逊,威斯康星州),一台瓦里安Mercury-VX300核磁共振光谱仪(Varian,Columbia, Maryland)和一个自动接触角仪(SL200A/B/D系列, Solon Tech. Inc. Ltd., Shanghai, China)。平均分子量是用凝胶渗透色谱仪(GPC)测量的,该仪器有一个Waters 2965D分离模块,一个Waters 2414折射率检测器,Shodex K802.5和K805色谱柱及Shodex K-G保护柱,聚苯乙烯标准品,以及以二甲基甲酰胺(DMF)为溶剂(流速为1.0mL/min;柱温为323K;检测器温度为323K)(Waters, Milford, Maryland)。NO.303结论:共聚碳酸盐P(DTC-co-PTC)是由DTC和PTC的微波辅助开环聚合合成的。分别使用Sn(Oct)2和Al(OiPr)3作为催化剂,通过微波辅助开环聚合DTC和PTC来合成。使用Pd/C催化剂进一步还原P(DTC-coPTC),产生部分脱保护的共聚碳酸盐HPDPC。与相应的P(DTC-co-PTC)相比,HPDPC显示出更快的降解率、更快的药物释放率和更多的亲水性。相应的P(DTC-co-PTC)。所有的共聚碳酸盐都具有稳定的药物释放率和良好的控释性能。
关闭-
1/9

-
2/9
还剩7页未读,是否继续阅读?
继续免费阅读全文产品配置单
北京祥鹄科技发展有限公司为您提供《开环共聚反应中微波辅助合成检测方案(微波萃取仪)》,该方案主要用于其他中其他检测,参考标准《暂无》,《开环共聚反应中微波辅助合成检测方案(微波萃取仪)》用到的仪器有电脑微波催化合成萃取仪 祥鹄XH-100A、微波固液相合成萃取仪。
我要纠错
推荐专场
相关方案










 咨询
咨询


