赛诺普Xenocs小角X射线散射仪研究溶液中BSA体系pH和蛋白质浓度对自身相互作用过程的影响
检测样品 其他
检测项目 蛋白质分子间的相互作用

 白金会员
57 篇解决方案
白金会员
57 篇解决方案
方案详情文
智能文字提取功能测试中
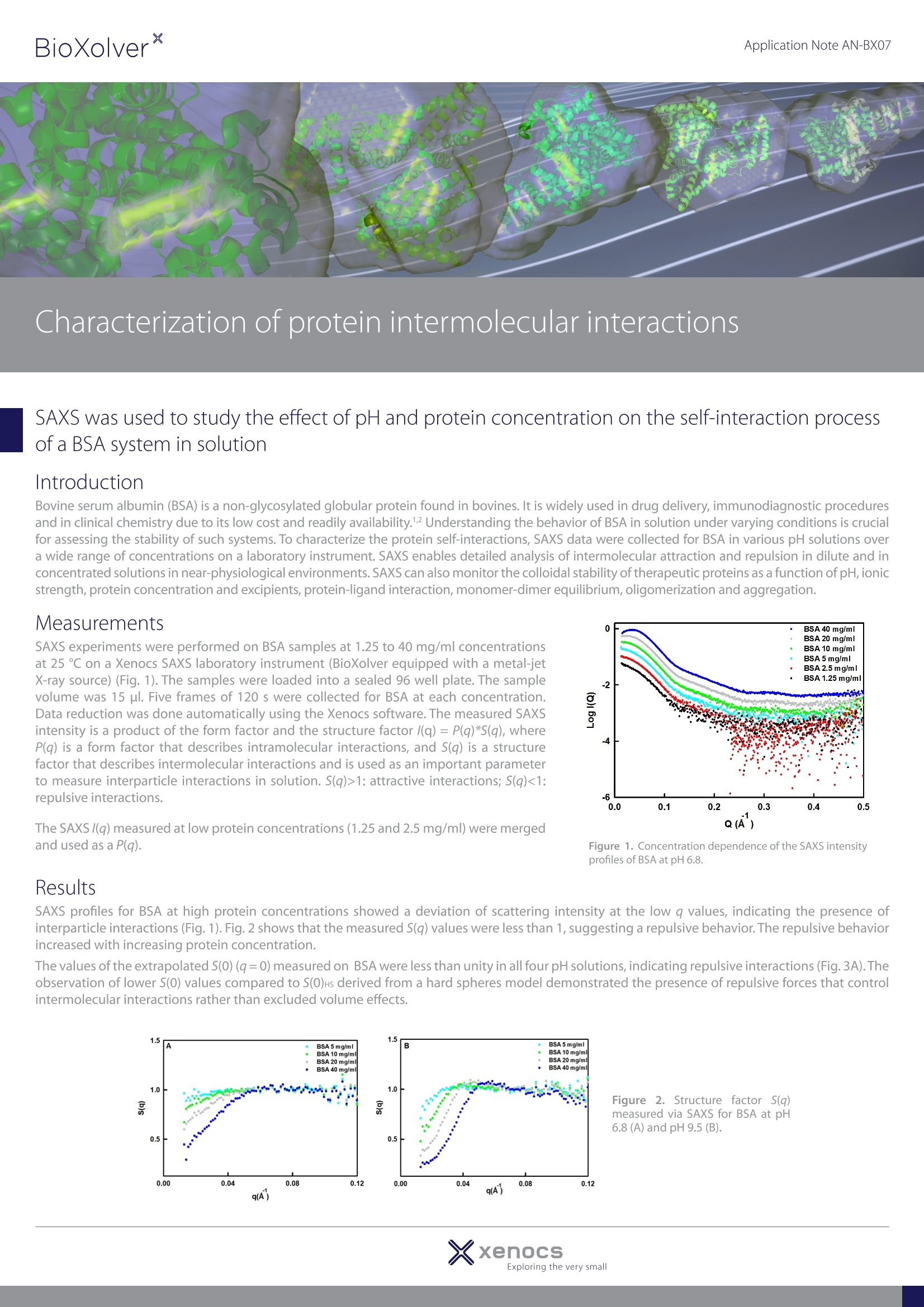
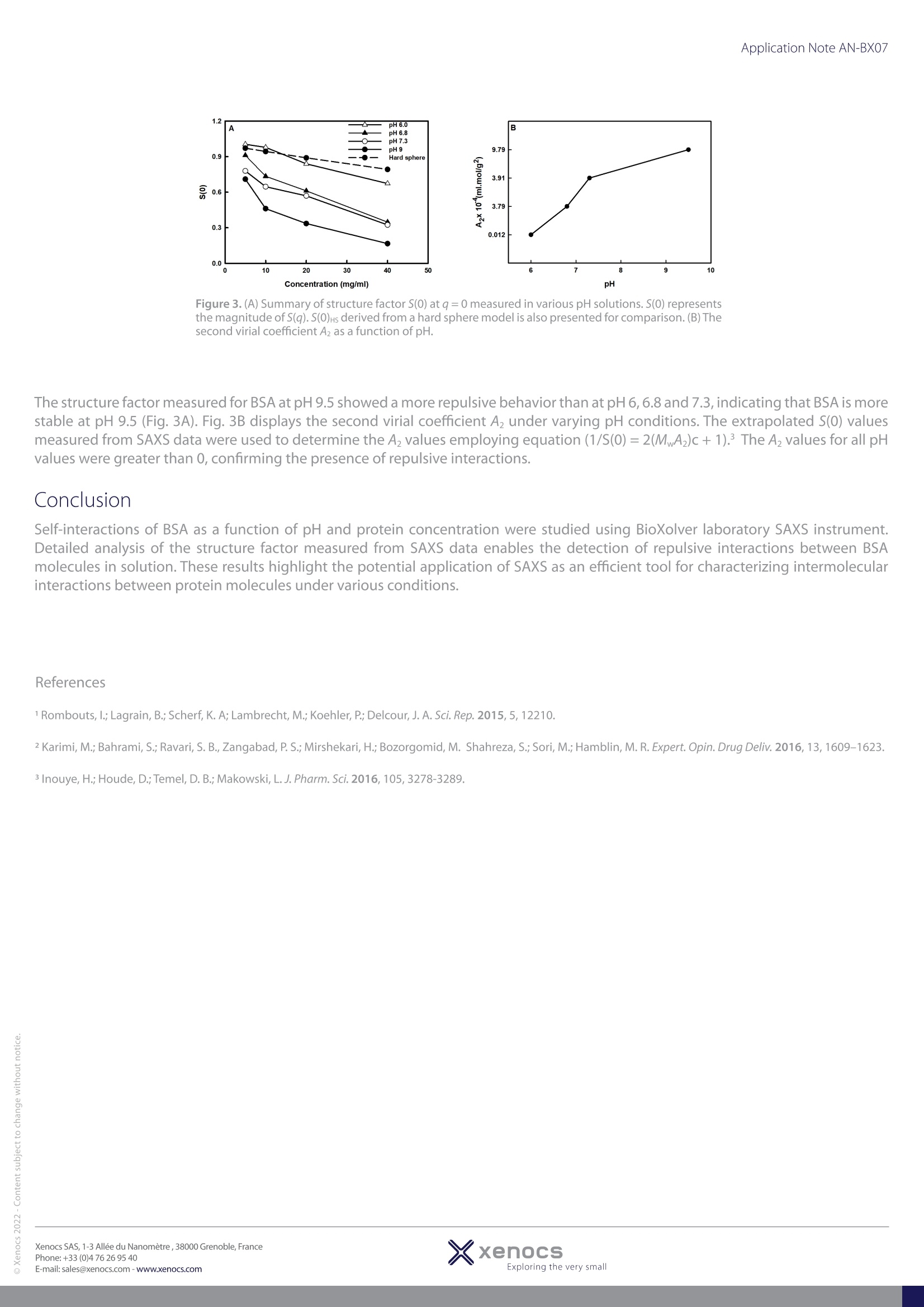
使用Xenocs公司SAXS实验室仪器(BioXolver配备metal-jet X射线光源),对浓度为1.25到40 mg/ml的BSA样品进行SAXS实验(图1)。样品被自动装载至密封的96孔盘上,样品量为15µl。每个浓度分别进行5次120秒采集。Xenocs软件可以自动完成数据还原。测得的SAXS强度是形态因子和结构因子I(q) = P(q)*S(q)的乘积,其中P(q)描述了分子内相互作用的形态因子,S(q)描述了分子间相互作用的结构因子,是测量溶液中粒子间相互作用的重要参数。 S(q)>1是吸引作用,S(q)>1是排斥作用。Application Note AN-BX07 Characterization of protein intermolecular interactions SAXS was used to study the effect of pH and protein concentration on the self-interaction processof a BSA system in solution Introduction Bovine serum albumin (BSA) is a non-glycosylated globular protein found in bovines. It is widely used in drug delivery, immunodiagnostic proceduresand in clinical chemistry due to its low cost and readily availability.Understanding the behavior of BSA in solution under varying conditions is crucialfor assessing the stability of such systems. To characterize the protein self-interactions, SAXS data were collected for BSA in various pH solutions overa wide range of concentrations on a laboratory instrument. SAXS enables detailed analysis of intermolecular attraction and repulsion in dilute and inconcentrated solutions in near-physiological environments. SAXS can also monitor the colloidal stability of therapeutic proteins as a function of pH,ionic strength, protein concentration and excipients, protein-ligand interaction, monomer-dimer equilibrium, oligomerization and aggregation. SAXS experiments were performed on BSA samples at 1.25 to 40 mg/ml concentrationsat 25 ℃ on a Xenocs SAXS laboratory instrument (BioXolver equipped with a metal-jetX-ray source) (Fig. 1). The samples were loaded into a sealed 96 well plate. The samplevolume was 15 ul. Five frames of 120 s were collected for BSA at each concentration.Data reduction was done automatically using the Xenocs software. The measured SAXSintensity is a product of the form factor and the structure factor /(q)= P(q)*s(q), whereP(q) is a form factor that describes intramolecular interactions, and S(q) is a structurefactor that describes intermolecular interactions and is used as an important parameterto measure interparticle interactions in solution. S(q)>1: attractive interactions; S(q)<1:repulsive interactions. Figure 1. Concentration dependence of the SAXS intensityprofiles of BSA at pH 6.8. Figure 3. (A) Summary of structure factor S(0) at q=0 measured in various pH solutions. S(0) representsthe magnitude of S(q). S(0)Hs derived from a hard sphere model is also presented for comparison.(B) Thesecond virial coefficient Az as a function of pH. The structure factor measured for BSA at pH 9.5 showed a more repulsive behavior than at pH 6, 6.8 and 7.3, indicating that BSA is morestable at pH 9.5 (Fig. 3A). Fig. 3B displays the second virial coefficient Az under varying pH conditions. The extrapolated S(0) valuesmeasured from SAXS data were used to determine the A2 values employing equation (1/S(0)=2(MA2)c+1).3 The Az values for all pHvalues were greater than 0, confirming the presence of repulsive interactions. Conclusion Self-interactions of BSA as a function of pH and protein concentration were studied using BioXolver laboratory SAXS instrument.Detailed analysis of the structure factor measured from SAXS data enables the detection of repulsive interactions between BSAmolecules in solution. These results highlight the potential application of SAXS as an efficient tool for characterizing intermolecularinteractions between protein molecules under various conditions. ( References ) 1 Rombouts, I.; Lagrain, B.; Scherf, K. A; Lambrecht, M.; Koehler, P.; Delcour, J. A. Sci. Rep. 2015,5,12210. 2 Karimi, M.; Bahrami, S.; Ravari, S. B.,Zangabad, P. S.; Mirshekari, H.; Bozorgomid,M. Shahreza,S.; Sori, M.; Hamblin, M. R. Expert. Opin. Drug Deliv. 2016, 13,1609-1623. 3 Inouye, H.; Houde, D.; Temel, D. B.; Makowski, L. J. Pharm. Sci. 2016,105,3278-3289. xenocsExploring the very small XxenocsExploring the very smallXenocs SAS, - Allee du Nanometre,Grenoble,FrancePhone:+() -mail: sales@xenocs.com-www.xenocs.com
关闭-
1/2
-
2/2
产品配置单
赛诺普(苏州)科学仪器有限公司为您提供《赛诺普Xenocs小角X射线散射仪研究溶液中BSA体系pH和蛋白质浓度对自身相互作用过程的影响》,该方案主要用于其他中蛋白质分子间的相互作用检测,参考标准《暂无》,《赛诺普Xenocs小角X射线散射仪研究溶液中BSA体系pH和蛋白质浓度对自身相互作用过程的影响》用到的仪器有Xenocs生物小角X射线散射仪BioXolver、赛诺普 BioXolver移液机器手。
我要纠错
相关方案




 咨询
咨询


