方案详情文
智能文字提取功能测试中
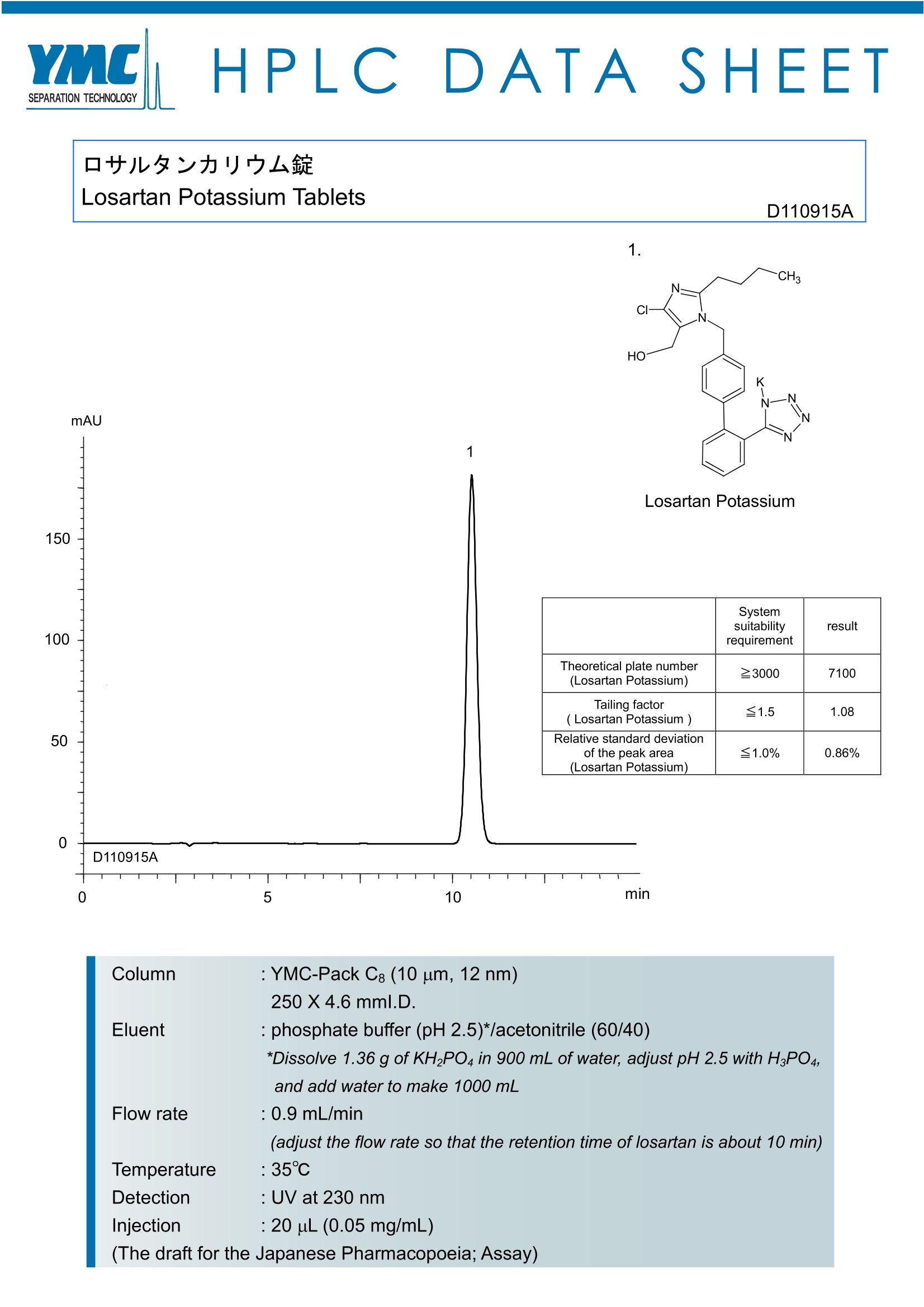
HPLC DATASHEET1 口艹儿夕力少中么锭Losartan Potassium Tablets D110915A 1. mAU Losartan Potassium Systemsuitability requirement result Theoretical plate number(Losartan Potassium) 23000 7100 Tailing factor( Losartan Potassium ) ≤1.5 1.08 Relative standard deviationof the peak area(Losartan Potassium) ≤1.0% 0.86% 0 5 10 min Column : YMC-Pack C: (10 um, 12 nm) 250X4.6mml.D. Eluent : phosphate buffer (pH 2.5)*/acetonitrile (60/40) *Dissolve 1.36 g of KH2PO4 in 900 mL of water, adjust pH 2.5 with HsPO4, and add water to make 1000 mL Flow rate : 0.9 mL/min (adjust the flow rate so that the retention time of losartan is about 10 min) Temperature :35℃ Detection : UV at 230 nm Injection :20 uL (0.05 mg/mL) (The draft for the Japanese Pharmacopoeia; Assay) 氯沙坦钾片含量测定色谱条件(JP) 仪 器:液相色谱仪检测器:UV (230nm)色谱柱:YMC-Pack C8色谱柱(内径:4.6mm 长度:250mm 粒径:10微米)流动相:乙腈-磷酸盐缓冲溶液* 40:60 *缓冲溶液:将1.36克磷酸二氢钾溶于900ml水中,并用磷酸调pH至2.5,再加水稀释至1000ml柱 温:35℃流 速:1.0ml/min(调整氯沙坦色谱峰保留时间约10分钟)进样量:20ul(0.05mg/ml)
关闭-
1/1
产品配置单
深圳凯米斯科技有限公司为您提供《氯沙坦钾片中主要物质含量检测方案(液相色谱柱)》,该方案主要用于化药制剂中含量测定检测,参考标准《暂无》,《氯沙坦钾片中主要物质含量检测方案(液相色谱柱)》用到的仪器有null。
我要纠错
相关方案


 咨询
咨询





