方案详情文
智能文字提取功能测试中
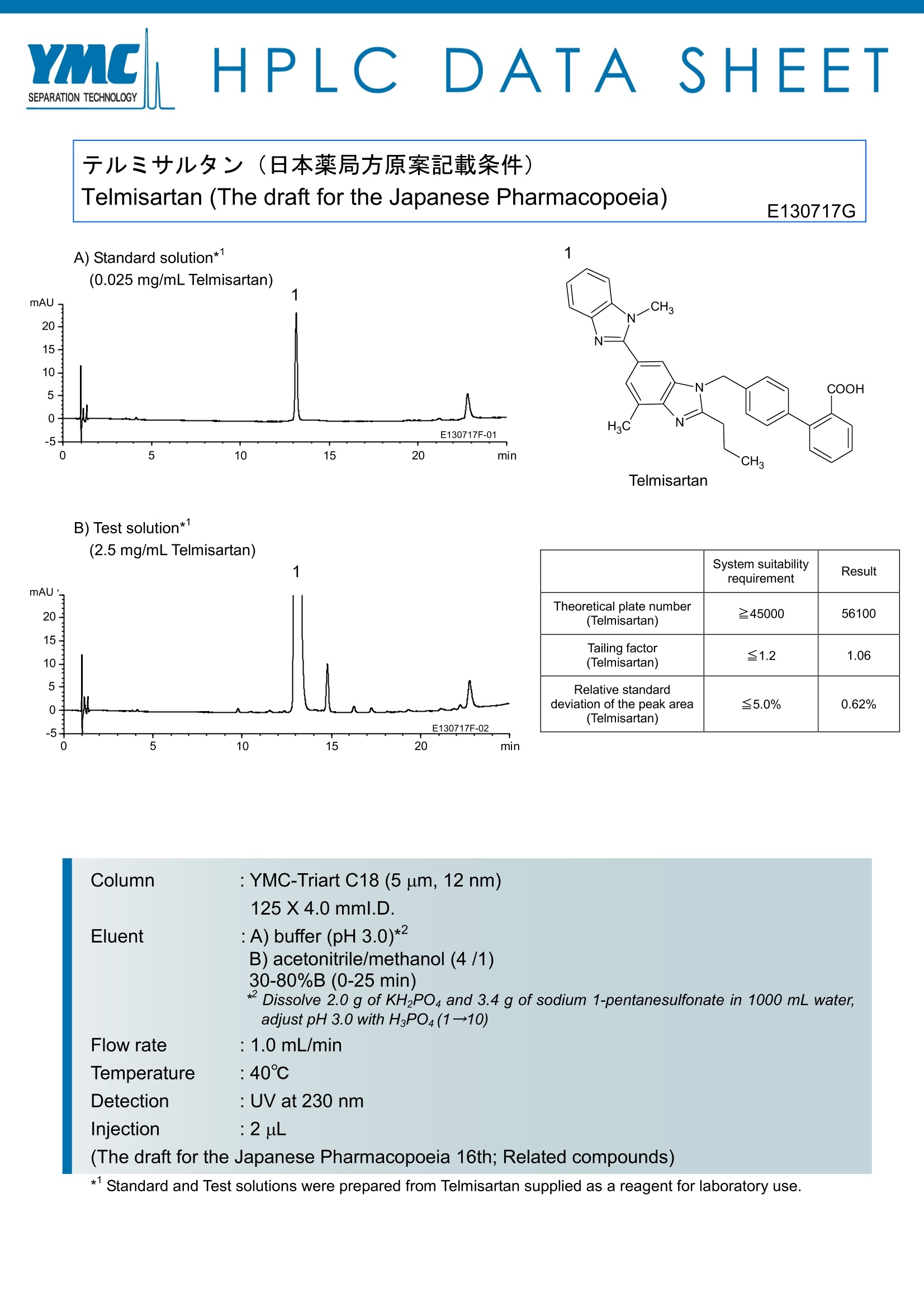
HPLC DATA :SHEET 于儿三艹儿夕>(日本薬局方原案記載条件)Telmisartan (The draft for the Japanese Pharmacopoeia) E130717G A) Standard solution*' Telmisartan B) Test solution**1(2.5 mg/mL Telmisartan) System suitabilityrequirement Result Theoretical plate number(Telmisartan) 245000 56100 Tailing factor(Telmisartan) ≤1.2 1.06 Relative standarddeviation of the peak area(Telmisartan) ≤5.0% 0.62% Column YMC-Triart C18 (5 um, 12 nm) 125X4.0 mml.D. Eluent :A) buffer (pH 3.0)* B) acetonitrile/methanol (4 /1) 30-80%B (0-25 min) *Dissolve 2.0 g of KH2PO4 and 3.4 g of sodium 1-pentanesulfonate in 1000 mL water, adjust pH 3.0 with H3PO4(1→10) Flow rate : 1.0mL/min Temperature : 40℃ Detection : UV at 230nm Injection :2uL (The draft for the Japanese Pharmacopoeia 16th; Related compounds) * Standard and Test solutions were prepared from Telmisartan supplied as a reagent for laboratory use. 替米沙坦有关物质检查的液相色谱条件色谱柱:YMC-Triart C18,5um, 4.0×125mm流动相:A)缓冲液 (pH3.0) B)乙腈-甲醇 (4/1) 梯度洗脱:30-80%B(0-25min)流速:1.0ml/min柱温:40℃检测器:UV(230nm)溶液浓度:对照液0.025mg/ml 测试液2.5mg/ml进样体积:2μL
关闭-
1/1
产品配置单
深圳凯米斯科技有限公司为您提供《替米沙坦中有关物质检测方案(液相色谱柱)》,该方案主要用于原料药中限度检查检测,参考标准《暂无》,《替米沙坦中有关物质检测方案(液相色谱柱)》用到的仪器有null。
我要纠错
相关方案


 咨询
咨询


