方案详情文
智能文字提取功能测试中
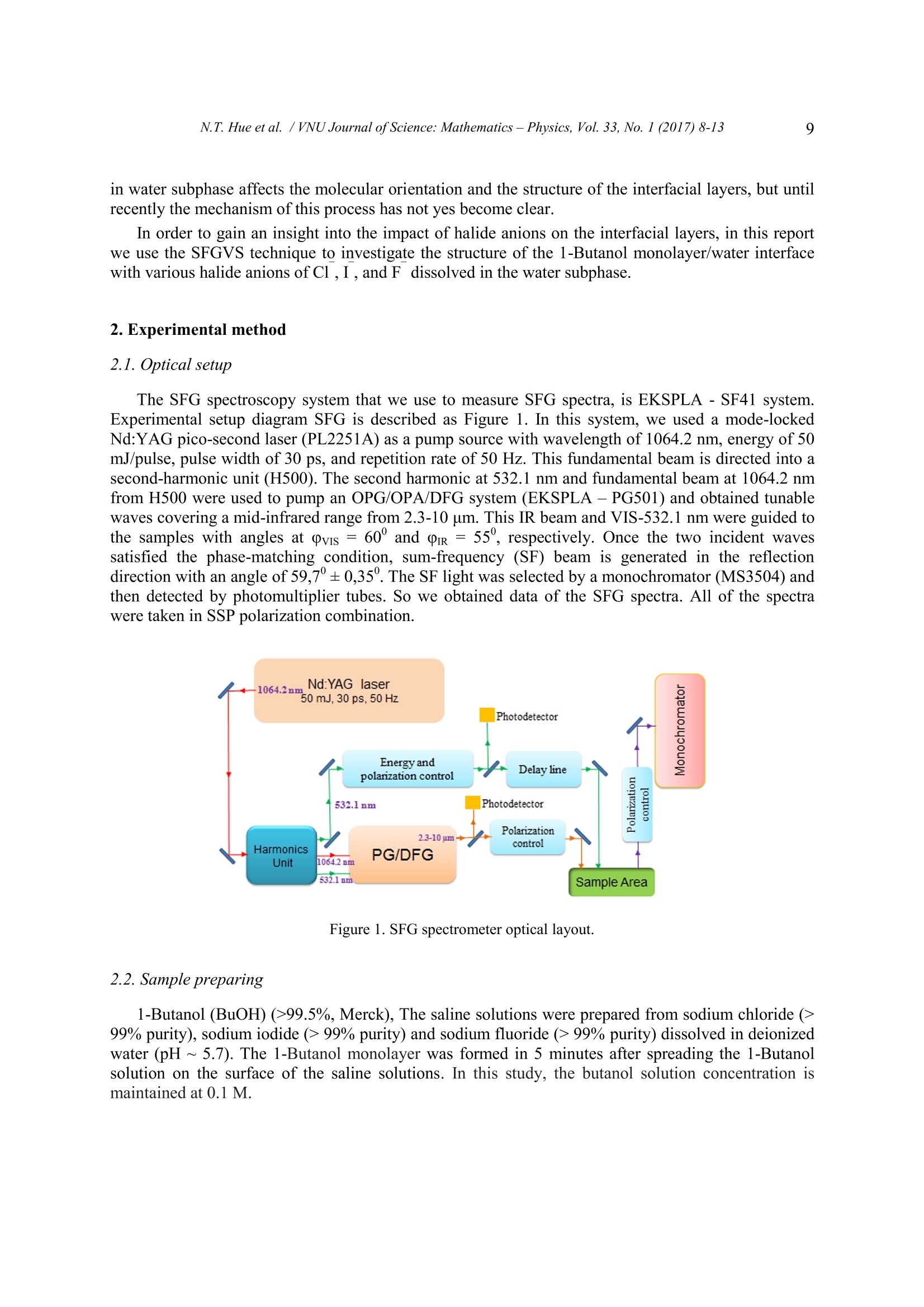
VNU Journal of Science: Mathematics -Physics, Vol. 33, No.1 (2017) 8-13 8 Effect of Halide Anions on Structure of 1-butanolMonolayer/water Interface Probed by Sum-frequencyVibrational Spectroscopy Nguyen Thi Hue, Vu Thi Thanh Tam', Nguyen Anh Tuanh* Department of Physics, VNU University of Science, Hanoi, VietnamHung Vuong University, Phu Tho Province, VietnamReceived 15 January 2017 Revised 16 February 2017; Accepted 20 March 2017 Abstract: In this report, we use a sum-frequency generation vibrational spectroscopy to measureSFG spectra from 1-Butanol monolayers on pure water and halide saline solution interfaces. Thesespectra indicate that halide anionshave different effects on the structure of 1-Butanolmonolayer/water interfaces. The obtained SFG spectra suggest that the I anions mostly disturbthe interfacial structure due to their largest surface propensity among those investigated. Keywords: Interfacial structure, Sum-frequency vibrational spectroscopy, 1-Butanol, halide salt. 1. Introduction Halide ions in seawater have been recognized as reactants in ozone depletion processes [1].However, a detailed effect of halogen ion on the water surface structure has not been suggested due tothe lack of tools to probe liquid surfaces specifically. As ultrafast laser technology has been developedrecently, the sum-frequency generation vibrational spectroscopy (SFGVS) gives not only staticinformation on surfaces but also dynamic properties of surface molecules [2]. Sum-frequency generation (SFG) is a second-order nonlinear optical process that has an intrinsicsensitivity to the struture at surfaces and interfaces. In the SFVS we can control the polarization ofboth two incident waves as well as the output (sum-frequency) wave. By choosing an appropriatepolarization combination for each SFG measurement we can also get the information of molecularorientation at the surface or interface. Molecules like fatty acids, lipids, and long-chain alcohols, which have both hydrophobic andhydrophilic parts, can form a Langmuir monolayer on water surface [3]. The structure of Langmuirmonolayers resembles biological membranes and becomes a good research model for 2-dimensionalsystems. Normally, the hydrogen bondings between the interfacial water molecules and thehydrophilic head groups help form the Langmuir monolayer. In general, the presence of dissolved ions ( C orresponding author. T el.: 84-919148855 ) ( Email: tuanphysics@vnu.edu.vn ) in water subphase affects the molecular orientation and the structure of the interfacial layers, but untilrecently the mechanism of this process has not yes become clear. In order to gain an insight into the impact of halide anions on the interfacial layers, in this reportwe use the SFGVS technique to investigate the structure of the 1-Butanol monolayer/water interfacewith various halide anions of Cl,I, and F dissolved in the water subphase. 2. Experimental method 2.1.Optical setup The SFG spectroscopy system that we use to measure SFG spectra, is EKSPLA -SF41 system.Experimental setup diagram SFG is described as Figure 1. In this system, we used a mode-lockedNd:YAG pico-second laser (PL2251A) as a pump source with wavelength of 1064.2 nm, energy of 50mJ/pulse, pulse width of 30 ps, and repetition rate of50 Hz. This fundamental beam is directed into asecond-harmonic unit (H500). The second harmonic at 532.1 nm and fundamental beam at 1064.2 nmfrom H500 were used to pump an OPG/OPA/DFG system (EKSPLA -PG501) and obtained tunablewaves covering a mid-infrared range from 2.3-10 um. This IR beam and VIS-532.1 nm were guided tcthe samples with angles at Qvis = 60' and Or= 55", respectively. Once the two incident wavessatisfied the phase-matching condition, sum-frequency (SF) beam is generated in the reflectiondirection with an angle of59,7°+0,35°. The SF light was selected by a monochromator (MS3504) andthen detected by photomultiplier tubes. So we obtained data of the SFG spectra. All of the spectrawere taken in SSP polarization combination. Figure 1.SFG spectrometer optical layout. 2.2. Sample preparing 1-Butanol (BuOH) (>99.5%,Merck), The saline solutions were prepared from sodium chloride (>99% purity), sodium iodide (> 99% purity) and sodium fluoride (>99% purity) dissolved in deionizedwater (pH~5.7). The 1-Butanol monolayer was formed in 5 minutes after spreading the 1-Butanolsolution on the surface of the saline solutions. In this study, the butanol solution concentration ismaintained at 0.1M. 3. Results and discussion We have taken SFG spectra from 1-Butanol monolayer on saline solutions such as NaI, NaCl, andNaF with various saline concentrations. In each SF spectrum of Fig.2, Fig.3, Fig.4, and Fig. 5 aredominated by the two prominent peaks at 2880 cm and 2940 cm’corresponding to a symmetricstretching mode of the methyl group (CH3ss) and CH, Fermi resonance (CH3Fr). The two weaklyobserved peaks at ~2855 cm° and 2905 cm°corresponding to a symmetric stretching mode of themethylene group (CH2ss) and CH Fermi resonance (CH2Fr) indicate that butanol molecules organizein all-trans conformations as predicted by other research [4]. Figure 2. SSP SFG spectra of1-Butanol/purewater interfaces. In Figure 2, the OH stretching band broaden 3000 cm° to 3600 cm is not stable because the 1-Butanol molecules of layers at the interface are dissolved in the water bulk. However, this band isstable as Fig. 3b, Fig.4b, and 5b. Such that the presence of halide salts in the beneath solutions canreduce the solubility of 1-Butanol, known as the salting out effect. Figure 3. SSP SFG spectra of 1-Butanol/NaF solutions. Figure 3 shows SFG spectra from the interface of the monolayer 1-Butanol/NaF solution withsalinity ranging from 0.0 to 0.9 M, which is close to the saturation of NaF. In Fig. 3a, the peakintensity of CHss mode from the 1-Butanol monolayer/NaF solution is significantly enhanced incompare to that from monolayer 1-Butanol/pure-water. The enhancement of this mode indicates an increasing interfacial order in the system with the presence of NaF in the subphase solution [4]. Otherresearch, both in experiment and simulation [5, 6], show that the presence of F anions in the air/waterinterface enhances the hydrogen bonding network of the interfacial water layers. In another report ofour group, we have also found this effect of NaF on the enhancement in the OH vibrational band froman Arachidic acid (AA) monolayer/water system. However, in the case of the AA monolayer/watersystem, we have observed an insignificant enhancement in the CH band. This result shows that the 1-BuOH monolayer is more sensitive to the ordering structure of the subphase interfacial water, and canbe a better indicator of that ordering, due to a shorter hydrocarbon chain length of the BuOH inrelative to the AA. We observe a contrary effect of NaI and NaCl on the interfacial structure of the 1-BuOHmonolayer/water system. In general, the presence of I and Clions in solutions decreases SFGsignals both in the CH and OH bands, as seen in Fig. 4, and Fig. 5. The lowering of CH3ss and CH3Fpeaks indicates a disorder in the interfacial structure of the systems. Meanwhile, the emergence of aCH2ss peak at 2850 cm, shown in figure Fig. 5a, indicates a collapsing of the 1-BuOH monolayerwhen the NaI concentration reaches to 3M. Results of phase-sensitive SFG spectroscopy research [7].and molecular dynamic simulation [5], showed a high surface propensity of Iand Cl ions onair/water interfaces. Due to the high surface propensity, I and Cl anions tend to disturb theinterfacial network structure of the 1-BuOH monolayer/water system. This argument is in goodagreement with SF spectra we have observed as the salt concentration increases, as indicated in Fig. 4and Fig.5. Figure 4. SSP SFG spectra of 1-Butanol/NaCl solutions. b) Figure 5. SSP SFG spectra of 1-Butanol/NaI solutions. The decrease of SF intensity in the OH vibrational band represents a disturbance in the hydrogen-bonding network of the interfacial water layers. Both I and Cl ions lower the SF signals from thisband, as seen in Fig. 4b, and Fig. 5b. However, when comparing between these two anions at the sameconcentration, as shown in Fig. 6, we observe different effects on the CH and the OH bands. In Fig.6a, the SF signal intensities of CH3ss and CH3Fr peaks from the 1-BuOH monolayer/NaCl solutionare larger than those from the 1-BuOH monolayer/NaI solution, whereas in Fig. 6b, the SF intensity ofOH band from the monolayer/NaCl solution is significantly lower than that from the monolayer/Nalsolution. Thus, the I ions have a stronger effect on the monolayer structure, whereas the Clionsmainly disturb the interfacial water layers beneath. This result is a supportive empirical evidencewhich shows that the surface propensity of Ianions is larger than that of Cl anions, as found inprevious studies on air/water systems [5, 7]. Figure 6. SSP SFG spectra of 1-Butanol/NaI and NaClsolutions. To qualitatively determine the order of the 1-BuOH monolayer structure, we evaluate the SFintensity ratio between the CH3ss and the CH2ss modes. The larger this ratio, the more order themonolayer is [4]. Those ratios for 2M and 3M concentrations of NaCl and NaI are given in table 1. Table 1. Calculated IcnIc ratios for the 1-BuOH/NaCl and NaI solutions Salt IcH/IcH, IcH, /IcH, Concentrations In NaCl In NaI 2 M 6.0 2.9 3 M 5.3 1.7 In table 1, the ratio Icn. /IcH. at the salt concentration of 2M of NaI is about half of that of NaCl.At the salt concentration of 3M, this ratio of NaI is only about one-third of NaCl, at which themonolayer is nearly collapsed under the disturbance of Iions. This evaluation is quite compatiblewith calculation results of an MD simulation study by Pavel Jungwirth et. al [5]. 4. Conclusions We have investigated the 1-Butanol monolayer/water interfacial system with various halide anionsconcentration in the water subphase using sum-frequency vibrational spectroscopy. We have observed a“structure-making”effect of F anions on the interfacial layers of the system, i.e. the SF signals fromthe interface are enhanced in the presence ofF anions. On the other hand, Cl and I anions disturbnetwork structures at the interface. Due to the larger surface propensity, iodide (I) has a moredisruptive effect on the monolayer, whereas chloride (Cl) has more disturbance on the hydrogenbonding network of the interfacial water beneath. References ( l[ 1 ] Roland v on Glasow, Sun, sea and ozone destruction, Nature, 453, (2008) 11 9 5-1196, ) ( [2] Y. R . Shen, S urface p roperties probed by second-harmonic and sum-frequency generation, Nature, 337 ( 1 989)519-525 ) ( [3] D. M yers, Surfaces, I nterfaces, and Colloids: Principle s and Applications, Wiley-VCH P ublishers, New York. 1999. ) ( [4] Lisa L. Van L oon, R e na N. Mi n or, an d Heather C. All e n, Structure of Bu t anol an d He x anol at A qu e ous,Ammonium Bisulfate, a n d Su l furic Acid Solution S u r faces I n vestigated by V i brational Sum Fr e quencyGeneration Spectroscopy, J. Phys. Chem. A, (2007), 111, p7338-7346. ) ( [5] P avel Jungwirth, a nd Douglas J . Tobias, Ions at t he Air/Water Interface, J. Phys. Chem. B , 2 002, 106, p 6361- 6373. ) ( [6] Elizabeth A. Raymond and Geraldine L . Richmond, Probing the Molecular Structure and B o nding of the Surfaceof Aqueous Salt S olutions, J. Phys. Chem. B, 108 (2004)5051-5059. ) ( [7] Chuanshan T i an, S t even J . B y rnes,Hui-Ling Han, an d Y. Ron Shen, Surface Prop e nsities of Atmospherically Relevant I ons in SaltSolutions Revealed b y Phase-Sensitive Su m Frequency Vibra t ionalSpectroscopy, Journa Physical Chemistry Letters 2 (2011) 1946. ) Halide ions in seawater have been recognized as reactants in ozone depletion processes [1]. However, a detailed effect of halogen ion on the water surface structure has not been suggested due to the lack of tools to probe liquid surfaces specifically. As ultrafast laser technology has been developed recently, the sum-frequency generation vibrational spectroscopy (SFGVS) gives not only static information on surfaces but also dynamic properties of surface molecules [2]. Sum-frequency generation (SFG) is a second-order nonlinear optical process that has an intrinsic sensitivity to the struture at surfaces and interfaces. In the SFVS we can control the polarization of both two incident waves as well as the output (sum-frequency) wave. By choosing an appropriate polarization combination for each SFG measurement we can also get the information of molecular orientation at the surface or interface. Molecules like fatty acids, lipids, and long-chain alcohols, which have both hydrophobic and hydrophilic parts, can form a Langmuir monolayer on water surface [3]. The structure of Langmuir monolayers resembles biological membranes and becomes a good research model for 2-dimensional systems. Normally, the hydrogen bondings between the interfacial water molecules and the hydrophilic head groups help form the Langmuir monolayer. In general, the presence of dissolved ions in water subphase affects the molecular orientation and the structure of the interfacial layers, but until recently the mechanism of this process has not yes become clear. In order to gain an insight into the impact of halide anions on the interfacial layers, in this report we use the SFGVS technique to investigate the structure of the 1-Butanol monolayer/water interface with various halide anions of Cl¯, I¯, and F¯ dissolved in the water subphase. affects the molecular orientation and the structure of the interfacial layers, but until recently the mechanism of this process has not yes become clear. In order to gain an insight into the impact of halide anions on the interfacial layers, in this report we use the SFGVS technique to investigate the structure of the 1-Butanol monolayer/water interface with various halide anions of Cl¯, I¯, and F¯ dissolved in the water subphase.
关闭-
1/6

-
2/6
还剩4页未读,是否继续阅读?
继续免费阅读全文产品配置单
北京欧兰科技发展有限公司为您提供《1-丁醇单层/水界面中和频振动光谱(SFG)检测方案(其它光谱仪)》,该方案主要用于其他中其他检测,参考标准《暂无》,《1-丁醇单层/水界面中和频振动光谱(SFG)检测方案(其它光谱仪)》用到的仪器有Ekspla SFG 表面和频光谱分析系统、PL2250 闪光灯泵浦皮秒Nd:YAG激光器。
我要纠错
相关方案



 咨询
咨询



