方案详情文
智能文字提取功能测试中
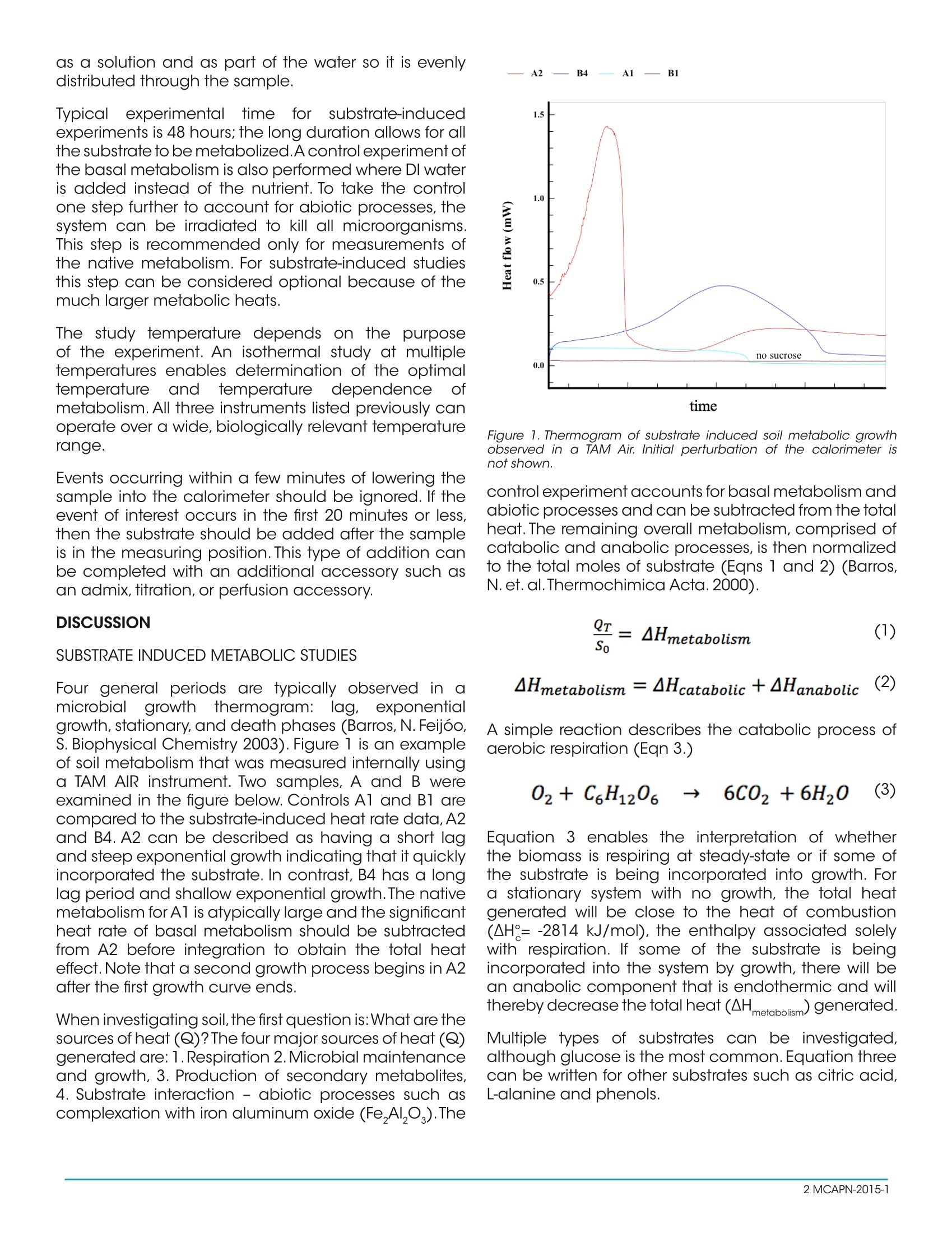
Hot Holobionts! Using Calorimetryto Characterize These Relationships ABSTRACT Isothermal calorimetry is a viable technique fordetermining the quality of soil through respirationmeasurements. Substrate induced respiration followsthe carbon in the soil and can be used to determinecarbon incorporation.. Additionalexperimentalconditions can further characterize the soil through theidea of calorespirometry. The findings via calorimetryin the references show a direct correlation betweencalorimetry results and soil quality. INTRODUCTION METABOLISM AND THE CARBON CYCLE Plants are a holobiont that include the rhizome andendophytes. This portion of the entire entity largelyinteracts with the soil environment which includesnutrients and biomass existing in the soil. Evaluation ofthe soil quality gives insight to the possibility ot plantquality or survival in a particular system. In a recentScience article these microbes and endophytes aredescribed as little farmhands and their symbioticrelationship with plants is likened to the microbiomeof the gut (de Vrieze, J Science, 2015). As the gutmicrobiome is currently being exploited, potential liesin exploration of the plant microbiome. Environmental quality is linked to carbon in the soil andin particular, areas with low soil organic matter (SOM)may have an increased risk of desertification (Barros,N. et al. Thermochimica Acta 2000). SOM contains thelargest pool of carbon in the terrestrial carbon cycleand accounting for this carbon and tracking its usehas strong environmental implications as well as plantlongevity (Herrman, A. et al. Environmental Science &Technology). A first approach would be to understandthe current environment and the next step wouldbe to tune the system to increase crop production,resistivity to pesticides, and investigate crops withspecific endophytes. Initial lab investigations could bean effective compliment to the field first approach"in understanding and designing a combination ofmicrobes, and biopesticides, and temperatures.All ofthese objectives can be probed via calorimetry. This paper discusses the1 Wuseofmetabolicmeasurements to describe microbial soil activity. Thisactivity is associated with particular environments such as deforestation that is known for poor organic matterbecause there is a connection between biomassand activity in terms of thermal efficiency. Althoughmetabolism consists of thousands of processes, thenet process can be simplified to the heat effects shownin the scheme below. The metabolic heat measuredcomes from two processes, catabolism that releasesenergy as heat (Q) and anabolism that requiresenergy.Abiotic processes usually produce negligibleheat since these are very slow processes. Scheme 1:Terrestrial Carbon Cycling. Interpreted from: Herrmann, A.,Coucheney, E. andNunan, N. "/sothermal Microcalorimetry ProvidesNew Insight into Terrestrial Carbon Cycling"Environmental Science &Technology.2014,48,4344-4352 ISOTHERMAL CALORIMETRY Isothermal calorimeters measure heat released orconsumed by a sample at a constant temperature.Heat is not used to induce these processes and theduration and temperature are the variables that theuser can control. For typical metabolic studies, aTAM Air, TAM I/lV or Multi Cell DSC can all be usedin isothermal mode. The calorimeter choice dependson the magnitude of the heat signal. For metabolicstudies on unamended soil, the TAM II/IV is the bestchoice. If the heat measured is from substrate-inducedmetabolism then the TAM Air and Multi Cell DSC willhave sufficient sensitivity. EXPERIMENTAL A common consideration for sample preparation is tohave sufficient head space in the ampoule to provideenough oxygen to support metabolism. For a 20 mLampoules,5 g dry weight of sample is a good startingpoint - this ratio can be scaled for smaller ampoules.The soil must be brought to a known fraction of thewater holding capacity with 60% being a goodplace to begin. The nutrient or substrate is delivered as a solution and as part of the water so it is evenlydistributed through the sample. Typical experimental time for substrate-inducedexperiments is 48 hours; the long duration allows for allthe substrate to be metabolized.A control experiment ofthe basal metabolism is also performed where DI wateris added instead of the nutrient. To take the controlone step further to account for abiotic processes, thesystem can be irradiated to kill all microorganisms.This step is recommended only for measurements ofthe native metabolism. For substrate-induced studiesthis step can be considered optional because of themuch larger metabolic heats. The study temperature depends on the p seof the experiment. An isothermal study at multipletemperatures enables determination of the optimaltemperatureand tempeririamatnuter e| dependence ofmetabolism. All three instruments listed previously canoperate over a wide, biologically relevant temperaturerange. Events occurring within a few minutes of lowering thesample into the calorimeter should be ignored. If theevent of interest occurs in the first 20 minutes or less,then the substrate should be added after the sampleis in the measuring position.This type of addition canbe completed with an additional accessory such asan admix,titration, or perfusion accessory. DISCUSSION SUBSTRATE INDUCED METABOLIC STUDIES Four general periods are typically observed in amicrobial growththermogram:lag, exponentialgrowth, stationary, and death phases (Barros,N. Feijoo,S. Biophysical Chemistry 2003). Figure 1 is an exampleof soil metabolism that was measured internally usinga TAM AIR instrument. Two samples, A and B wereexamined in the figure below. Controls A1 and B1 arecompared to the substrate-induced heat rate data, A2and B4. A2 can be described as having a short lagand steep exponential growth indicating that it quicklyincorporated the substrate. In contrast, B4 has a longlag period and shallow exponential growth.The nativemetabolism for A1 is atypically large and the significantheat rate of basal metabolism should be subtractedfrom A2 before integration to obtain the total heateffect.Note that a second growth process begins in A2after the first growth curve ends. When investigating soil, the first question is: What are thesources of heat (Q)?The four major sources of heat (Q)generated are:1.Respiration 2. Microbial maintenanceand growth, 3. Production of secondary metabolites,4. Substrate interaction - abiotic processes such ascomplexation with iron aluminum oxide (Fe,AI,O).The time Figure 1. Thermogram of substrate induced soil metabolic growthobserved in a TAM Air. Initial perturbation of the calorimeter isnot shown. control experiment accounts for basal metabolism andabiotic processes and can be subtracted from the totalheat.The remaining overall metabolism, comprised ofcatabolic and anabolic processes, is then normalizedto the total moles of substrate (Eqns 1 and 2) (Barros,N. et.al.Thermochimica Acta.2000). A simple reaction describes the catabolic process ofaerobic respiration (Eqn 3.) Equation 3 enables the interpretation of whetherthe biomass is respiring at steady-state or if some ofthe substrate is being incorporated into growth. Fora stationary system with no growth, the total heatgenerated will be close to the heat of combustion(AH°=-2814 kJ/mol), the enthalpy associated solelywith respiration. If some of the substrate is beingincorporated into the system by growth, there will bean anabolic component that is endothermic and willthereby decrease the total heat (AH metabolism)generated. Multiple types of substrates can be investigated,although glucose is the most common. Equation threecan be written for other substrates such as citric acid,L-alanine and phenols. ASSIGNING ENTHALPY Substrate-induced samples that have higher rates ofglucose degradation and microbial growth dissipateless energy as heat, retain more energy in the system,and have higher biomass yield. If no biomass iisforming,i.e. there is no growth, then all the heat is lostto combustion/respiration. The von Stockar energy balance can be used todescribe the system (Eqn 4) (von Stockar, U., Liu, J.Biochim. Biophys Acta.1999). After this mass balance equation, the conservationof glucose can be described by the followingrelationships (Eqn 5-7, Barros, N. Feijoo, S., Alvarez, J.International Journal of Low Carbon Technologies).AH0 is the enthalpy change for the microbial growthreaction, Yys is the growth yield where X is moles ofbiomass and S is moles of glucose. Finally, AH9 isthe enthalpy change for the cons1ierrYatiarvation glucosedegradation reaction and A H9 is the combustion ofthe nitrogen source, if applicable. It is added in thecase of Equation 4 and in other cases where the soilhas been stimulated by the addition of ammonium. Ifthis compound has not been added then this portionof the equation can be ignored. Determination of the soillbiomasss(X) canbedetermined via a few different methods. Two commonways the mass of living bacteria (X) can be determinedis by the colony forming units from extracted soil or bySparling's method (Sparling,G. Soil Biol. Biochem 1981and Sparling, G.P. J. Soil Sci. 1983). The normalizedenthalpy and related values from the equations abovecan be further applied to determine the thermal yieldand the value Yys is correlated to the efficiency of thesystem in degrading soil organic matter. CALORESPIROMETRY Calorespirometryiss an approach for assessingmicrobial efficiency in soils. It couples the simultaneousmeasurement of CO, (Rco2)and heat (R.) productionratesby isisothermal calorimetry. The additionalinformation on co, increases understanding anddescription of the soil system (Figure 2). Figure 2. Multi-dimensional plot displacing a relationship betweenvariables of soil metabolism. This figure was created by Serita Frey(UNH), unpublished data. The ratio of heat to Co, production is related tometabolic efficiency (Eqn 8, Barros, N. et al J. ThermAnal Calorim. 2010). e is the metabolic efficiency, Ys is the oxidation state ofsubstrate C and y, is the oxidation state of microbialbiomass, which is assumed to be-0.3.△Ho,) isThornton’sconstant (-455±15 kJ/mol) and AH is the difference inthe heat of combustion of the biomtiyelamss and glucose(-559 and -469 kJ/Cmol, respectively). There are afew ways that have been used to obtain the co, (g)released and these include,but are not limited to, sodalime capture, gel plate detector, and portable infraredgas analyzer. In addition to these ancillary methodsso mtfCO, rates can be quantified via calorimetry. A solutionof fresh concentrated, sodium hydroxide solution canbe added to the sample vial (Picture1). The excessheat released in the presence of sodium hydroxide isdirectly related to the rate of CO,(g) release; the heatof this reaction is -108.5 kJ/mol. To perform this type ofanalysis, the respiration rate should be taken with andwithout the NaOH present. Picture 1. A 20 mL reaction vial with asolution of NaOH placed into the chamberfor calorespiration determinations. The difference in the heat rates with NaOH and withoutNaOH gives the rate of production of CO, (Eqn 9). The integral of the plot of Rco, versus time givesthe moles of CO,. After this both the heat and gasexchange is measured and the metabolic efficiencyof the biomass is better understood. CONCLUSION Calorimetry provides adirect(qualitative andquantitativee(assessmentt ofsoil1 condition1 andcarbon cycling. In addition to heat measurements,simultaneous measurements of heat and co,production increase understanding of soil processes.Well-designed control experiments allow separationof measured heat into different sources. Trends incalorimetric evaluation of soil have emerged that areconsistent with soil conditions. This technique and theanalysis methods applied to soil can also be appliedto plants, insects, and other organisms. ACKNOWLEDGEMENTS Thispaper waiass authored by Colette Quinn,AInstruments. TA Instruments would like to thank LeeHansen (Brigham Young University) and Nieves Barros(Universit/ yO fof Santiago de Compostela) for theirdirection through material and discussion. REFERENCES GENERAL MICROBIAL GROWTH THERMODYNAMICS ·Braissant, O.Wirz, D., Gopfert, B., Daniels, A. "Use ofisothermal microcalorimetry to monitor microbialactivities" FEMS Microbiol.Lett.2010,303:1-8. ( · d e Vrieze,J.The Littlest Farmhands"Science.349:680- 683. ) SOIL THERMODYNAMICS ( ·Barros,N.,Feijoo,S.,Fernandez,S.,Airoldi,S.Applicationof the metabolic enthalpy change in s tudies ofsoil microbioal activity" Thermochimica A c ta. 2000, 356:1-7. ) ( · Barros, N., Feijoo, S., Alverez, J.A thermodynamic model and software t o a s sess t h e contribution of land u se to global warming"International Journal ofLow Carbon Technologies.3/1 ) ·Barros,N., Feijoo, S. A combinedmassisandenergy balance to provide bioindicators of soilmicrobiological quality" Biophysical Chemistry. 2003,104:561-572. · Barros,N.,Hansen,L.Pineiro,V.,Vikegard,P"Calorimetrymeasuresthe response of soil organic matterbiodegradation to increasing temperature" Journalof Thermal Analysis and Calorimetry. 2015. Publishedonline 8/8/2015. ( · H arris,J.,Ritz,K.,Coucheney,E.,Grice,S.,Lerch,T.,Pawlett,M., Herrmann, A. "The thermodynamic efficiencyof soil microbial communities subject t o l o ng-termstress i s lower than those under conventional inputregimes" Soil Biology & Biochemistry.2012,47:149-157. ) · Herrmann, A., Coucheney, E., Naoise, N. "IsothermalMicrocalorimetry Provides New Insight into terrestrialCarbon1 Cycling"Environmental Science &Technology. 2014,48:4344-4352 · Plante, A., Fernandez, J., Leifeld, J."Applicationsof thermal analysis techniques in soil science"Geoderma.2009.153:1-10. ·Von Stockar, U., Liu, J. "Does microbial life alwaysfeed on negative entropy? Thermodynamic analysisof microbial growth" Biochim. Biophys Acta. 1999,1412:191-211. ·Wadso, I. "Characterization of microbial activity insoil by use of isothermal microcalorimetry".J.Therm.Anal. Calorim.2009.95:843-850. BASAL METABOLIC STUDY ( · Barros, N., Gallego, M ., F e ijoo, S . " S ensitivity of calorimetric indicators of s o il m i crobial activity" Thermochimica Acta.2007,458:18-22. ) CALORESPIROMETRY ( ·Barros, N. Feijoo,S.; H ansen, L . Ca lorimetric determination of metabolic heat, CO2 r ates and calorespirometric rati o of soil basal metabolism" Geoderma.2011,1 6 0:542-547. ) ( · B arros, N.,Salgado, J.,Rodrigues-Anon,A., P r oupin,J.,Villanueva, M., Hansen, L."Calorimetric approach to metabolic carbon conversion"J.Therm Anal Calorim. 2010.99:771-777. ) ·Barros, N., Merino, A., Martin, M., Perez, C., Hansen,L. Changes in soil organic matter in a forestrychronosequence monitored by thermal analysis andcalorimetry"Spanish Journal of Soil Science. 2014, 4:239-253. ·Nogales,A., Munoz-Sanhueza, L. Hansen, L, Arnholdt-Schmitt "Phenotypcing carrot (Daucus carota L.)for yield-determining temperature rreesponse bycalorespriometry"Planta.2015, 241:525-538. BIOMASS DETERMINATION ( · Kimura, T., Takahashi. K. "Calorimetric s tudies of soil microbes: quantitative relation between h eatevolution during microbial degradation o f g lucoseand changes in microbioal activity in s oil"J. Gen. Microbiol.1985,131:3083-3089. ) ( · T issot, P."Calorimetric study of th e bioremediation ofa polluted soil"J.Thermal Anal.Cal. 1999,57:303-312. ) · Sparling G.P. (1981) Microcalorimetry and othermethods to assess biomass and activity in soil. SoilBiol.Biochem.13:93-98 · Sparling, G.P."Estimation of microbial biomass andactivity in Ds0oil using microcalorimetry"J.Soil Sci.1983,34:381-390. MCAPN- Isothermal calorimetry is a viable technique for determining the quality of soil through respiration measurements. Substrate induced respiration follows the carbon in the soil and can be used to determine carbon incorporation. Additional experimental conditions can further characterize the soil through the idea of calorespirometry. The findings via calorimetry in the references show a direct correlation between calorimetry results and soil quality.
关闭-
1/5
-
2/5
还剩3页未读,是否继续阅读?
继续免费阅读全文产品配置单
TA仪器为您提供《微生物中代谢,生物量检测方案(差示扫描量热)》,该方案主要用于其他中代谢,生物量检测,参考标准《暂无》,《微生物中代谢,生物量检测方案(差示扫描量热)》用到的仪器有TA仪器+等温量热仪+TAM Air。
我要纠错
推荐专场
差示扫描量热仪(DSC/DTA)
更多相关方案






 咨询
咨询