NaCrO2中循环过程的电子结构和相变特性检测方案(顺磁共振波谱)
检测样品 电子元器件产品
检测项目 循环过程的电子结构和相变特性
方案详情文
智能文字提取功能测试中
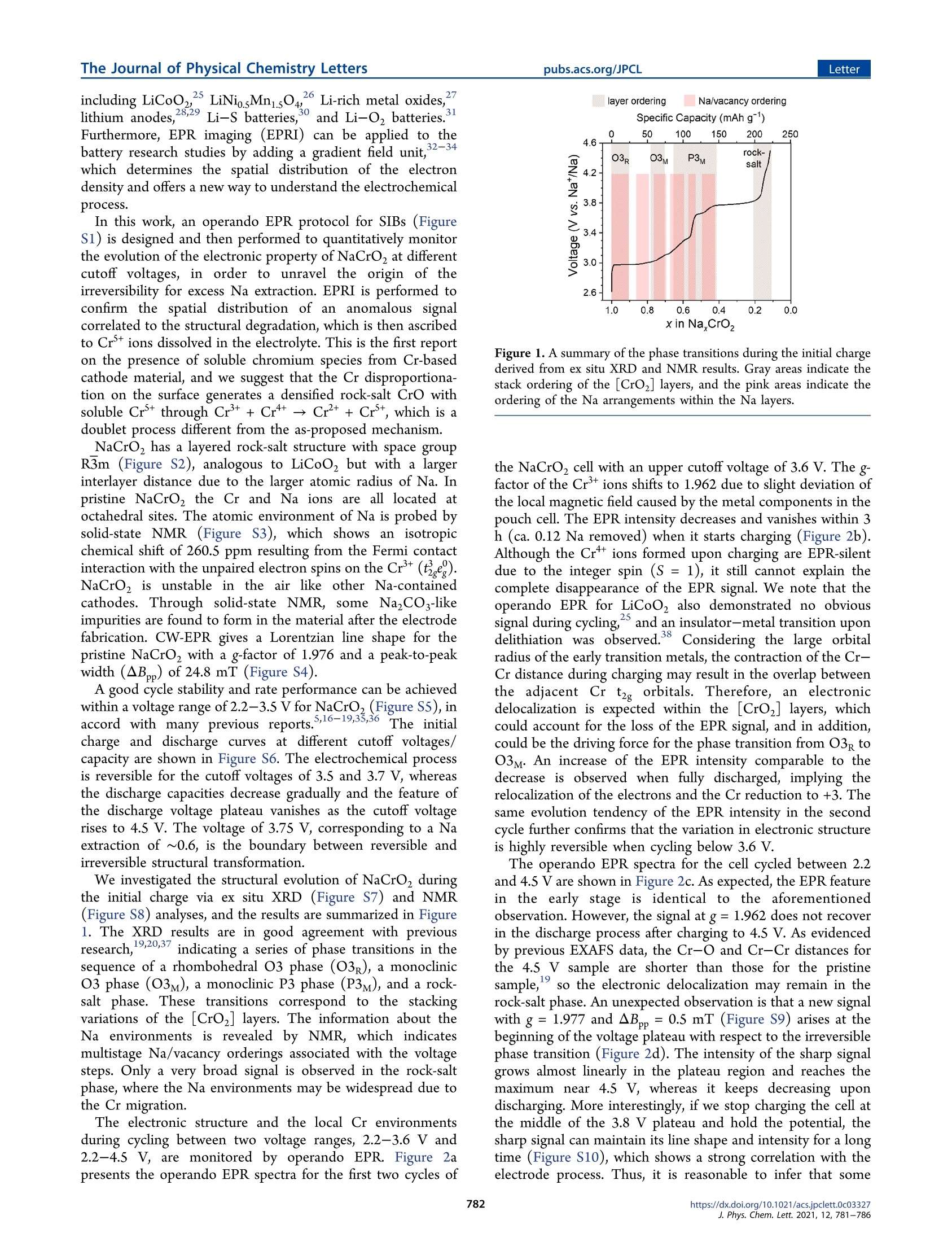
THE JOURNAL OFPHYSICAL CHEMISTRYLetter The Journal of Physical Chemistry LettersLetterpubs.acs.org/JPCL LETTERS AJOURNALOF THE AMERICAN CHEMICAL SOCIETY pubs.acs.org/JPCL Operando EPR and EPR Imaging Study on a NaCrO, Cathode:Electronic Property and Structural Degradation with Cr Dissolution Fushan Geng, Qi Yang, Chao Li, Bei Hu, Chong Zhao, Ming Shen, and Bingwen Hu* Cite This: J. Phys. Chem. Lett. 2021, 12, 781-786 Read Online ACCESS| Metrics & More Article Recommendations sl Supporting Information ABSTRACT: NaCrO, is a potential cathode material for sodium-ion batteries due to its lowcost, safety, and high power. It is necessary to further understand its electronic propertyduring cycling in advance of practical application. In this work, operando EPR is carried outto monitor the evolution of the electronic structure for NaCrO2 cycled between 2.2-3.6Vand 2.2-4.5 V. We discover that electronic delocalization takes place at the early stage ofcharge, which may account for the excellent rate performance. In addition, via EPR imaging,an EPR signal associated with the irreversible phase transition at 3.8 V is located in theelectrolyte, which is then attributed to the Cr+ions dissolved with the surfacereconstruction. These findings may help researchers to better design and modify the Cr-based cathode materials. ith the upscaling of electric W.vehiclee production,reducing the battery cost especially on the cathodematerials is of great importance. In general, the cathodematerial is a metal oxide containing alkali metal ions asshuttling ions and transition metal ions as redox centers.Making use of inexpensive metal ions in the cathode istherefore a long-term goal for sustainable battery development.The shortage of Li resources has driven researchers to makemore effort on the sodium-ion battery (SIB) technology overthe past decade, although the sodium-based systems have acertain weakness in energy density compared to their lithiumequivalents due to the lower redox potential and their heavieratomic mass.3,4 Fortunately, the layered oxide cathodes for SIBs composedof inexpensive 3d transition metals (Cr, Mn, and Fe) have agreater cycle stability than their lithium equivalents,S-14 whichmakes it possible to employ SIBs on large vehicles and instationary energy storage from an economy perspective.Among them, NaCrO, is an extremely promising material forpractical application due to the following aspects. (i) NaCrO,shows high thermal stability in nonaqueous electrolyte at hightemperature, even better than delithiated LiFePO4(ii) TheCoulombic efficiency is higher than 90% for the first cycle andremains approximately 100%during long-time c48cycling,demonstrating few side reactions.(iii)Temperatureadaptability is wide, from -15 to 55 C.(iv) No largevariation in voltage curve is observed. And, (v) throughcarbon-coating, an extraordinary rate capability up to 150 Ccan be achieved, leading to full discharge within 27 s for a high-power scenario.18 However, the reversible capacity for NaCrO, is usuallylimited to ca. 120 mAh g, corresponding to ca.0.5 Na extraction in NaCrO, and the reversibility is quickly lost byfurther charge above the plateau of 3.8 V. For all we know,there were only two studies attempting to clarify the structuraldegradation mechanism for excess extraction of Na ions inNaCrO. Kubota and co-workers suggested the migration of Crions from the [CrO2] layers to both tetrahedral and octahedralsites in the Na layers upon charging over 3.8V, resulting in theirreversible phase transition with a drastic shrinkage ofinterslab space.Bo and co-workers further proved that thecapacity decay arises from a layered-to-rock-salt transformationwhen more than 0.6 Na is removed, during which the chargedisproportionation of Cr(3Cr*+→2Cr++ Cr) and thecomproportionation of Cr3+/6+ (2Cr++ Cr*+→3Cr) initiatethe Cr migration and a metastable intermediate O3 Na;CrO,phase is formed.2 Given that the disproportionation/comproportionation ofCr ions would be involved with complex electron transfer,electron paramagnetic resonance (EPR) spectroscopy, whichhas been extensively employed in different cathode materialresearch studies,21-24 is very competent to study such system.Moreover, due to the low energy of microwave radiation, real-time monitoring of the whole battery can be conducted in theEPR cavity with no radiation damage to the materials. Manyremarkable findings have been made in various systems, ( Received: November 6, 2020 ) ( Accepted: J anuary 6, 2021 ) including LiCoO25 LiNisMn sO426 Li-rich metal oxides,lithium anodes,28,29 Li-S batteries, and Li-O, batteries.Furthermore, EPR imaging (EPRI) can be applied to thebattery research studies by adding a gradient field uniitt ,32-34which determines the spatial distribution of the electrondensity and offers a new way to understand the electrochemicalprocess. In this work, an operando EPR protocol for SIBs (FigureS1) is designed and then performed to quantitatively monitorthe evolution ofthe electronic property of NaCrO, at differentcutoff voltages, in order to unravel the origin of theirreversibility for excess Na extraction. EPRI is performed toconfirm the spatial1(distribution of an anomalous signalcorrelated to the structural degradation, which is then ascribedto Crions dissolved in the electrolyte. This is the first reporton the presence of soluble chromium species from Cr-basedcathode material, and we suggest that the Cr disproportiona-tion on the surface generates a densified rock-salt CrO withsoluble Crthrough Cr+ Cr*→Cr++ Crt, which is adoublet process different from the as-proposed mechanism. NaCrO, has a layered rock-salt structure with space groupR3m (Figure S2), analogous to LiCoO, but with a largerinterlayer distance due to the larger atomic radius of Na. Inpristine NaCrO the Cr and Na ions are all located atoctahedral sites. The atomic environment of Na is probed bysolid-state NMR (Figure S3), which shows an isotropicchemical shift of 260.5 ppm resulting from the Fermi contactinteraction with the unpaired electron spins on the Cr(te).NaCrO, is unstable in the air like other Na-containedcathodes. Through solid-state NMR, some NazCO3-likeimpurities are found to form in the material after the electrodefabrication. CW-EPR gives a Lorentzian line shape for thepristine NaCrO with a g-factor of 1.976 and a peak-to-peakwidth (AB) of 24.8 mT (Figure S4). A good cycle stability and rate performance can be achievedwithin a voltage range of 2.2-3.5 V for NaCrO, (Figure SS), inaccord with many previous reports.,16-1935,36TThe initialcharge and discharge curves atdifferent cutoff voltages/capacity are shown in Figure S6. The electrochemical processis reversible for the cutoff voltages of 3.5 and 3.7 V, whereasthe discharge capacities decrease gradually and the feature ofthe discharge voltage plateau vanishes as the cutoff voltagerises to 4.5 V. The voltage of 3.75 V, corresponding to a Naextraction of ~0.6, is the boundary between reversible andirreversible structural transformation. We investigated the structural evolution of NaCrO, duringthe initial charge via ex situ XRD (Figure S7) and NMR(Figure S8) analyses, and the results are summarized in Figure1. The XRD results are in good agreement with previousresearch, 19,20,37 indicating a series of phase transitions in thesequence of a rhombohedral O3 phase (O3p), a monoclinicO3 phase (O3M), a monoclinic P3 phase (P3M), and a rock-salt phase. These transitions correspond to the stackingvariations of the [CrOz] layers. The information about theNa environments is revealed by NMR, which indicatesmultistage Na/vacancy orderings associated with the voltagesteps. Only a very broad signal is observed in the rock-saltphase, where the Na environments may be widespread due tothe Cr migration. The electronic structure and the local Cr environmentsduring cycling between two voltage ranges, 2.2-3.6 V and2.2-4.5s V, are monitored by operando EPR. Figure 2apresents the operando EPR spectra for the first two cycles of Figure 1. A summary of the phase transitions during the initial chargederived from ex situ XRD and NMR results. Gray areas indicate thestack ordering of the [CrOz] layers, and the pink areas indicate theordering of the Na arrangements within the Na layers. the NaCrO, cell with an upper cutoff voltage of 3.6 V. The g-factor of the Crions shifts to 1.962 due to slight deviation ofthe local magnetic field caused by the metal components in thepouch cell. The EPR intensity decreases and vanishes within 3h (ca. 0.12 Na removed) when it starts charging (Figure 2b).Although the Crions formed upon charging are EPR-silentdue to the integer spin (S = 1), it still cannot explain thecomplete disappearance of the EPR signal. We note that theoperando EPR for LiCoO, also demonstrated no obvioussignal during cycling,and an insulator-metal transition upondelithiation was observed.8 Considering the large orbitalradius of the early transition metals, the contraction of the Cr-Cr distance during charging may result in the overlap betweenthe adjacent Cr t orbitals. Therefore, an electronicdelocalization is expected within the [CrOz] layers, whichcould account for the loss of the EPR signal, and in addition,could be the driving force for the phase transition from O3g toO3M. An increase of the EPR intensity comparable to thedecrease is observed when fully discharged, implying therelocalization of the electrons and the Cr reduction to +3. Thesame evolution tendency of the EPR intensity in the secondcycle further confirms that the variation in electronic structureis highly reversible when cycling below 3.6 V. The operando EPR spectra for the cell cycled between 2.2and 4.5 V are shown in Figure 2c. As expected, the EPR featurein the early stage is identical to the aforementionedobservation. However, the signal at g= 1.962 does not recoverin the discharge process after charging to 4.5 V. As evidencedby previous EXAFS data, the Cr-O and Cr-Cr distances forthe 4.5 V sample are shorter than those for the pristinesample, so the electronic delocalization may remain in therock-salt phase. An unexpected observation is that a new signalwith g= 1.977 and AB,p= 0.5 mT (Figure S9) arises at thebeginning of the voltage plateau with respect to the irreversiblephase transition (Figure 2d). The intensity of the sharp signalgrows almost linearly in the plateau region and reaches themaximum near 4.5 V, whereas it keeps decreasing upondischarging. More interestingly, if we stop charging the cell atthe middle of the 3.8 V plateau and hold the potential, thesharp signal can maintain its line shape and intensity for a longtime (Figure S10), which shows a strong correlation with theelectrode process. Thus, it is reasonable to infer that some Figure 2. Operando EPR spectra of the NaCrO,/Na cell cycled at 10 mAg-between (a) 2.2-3.6V and (c) 2.2-4.5V. The intensive Na signal inthe range of 331-335 mT is truncated for clarity. (b, d) Corresponding voltage profiles (top) and EPR intensities (bottom) as a function of time.EPR intensities are calculated using half of the peak-to-peak intensity. Figure 3. EPR images of the operando cell charged to 4.15 V on (a) the ZY plane and (b) the XY plane. The spectral width for EPR imaging islimited to the unknown signal. Color bars on the right side show the normalized intensity of spin concentrations. (c) Digital photos of the cell usedin EPR imaging. The sizes of the cathode and the separator are marked in blue and red, respectively. (d) Scheme for the orientation of the cell inthe image coordinates. The viewing direction is indicated by blue arrows. paramagnetic species are formed during the layered-to-rock-salt transformation. EPRI is then carried out on the operando cell aiming at theunknown signal in order to determine its spatial distribution.A low gradient field of 25 G cm- was employed here because thesignal-to-noise ratio (SNR) of the unknown signal is poor.Although a strong gradient field (e.g.,200 G cm-) could leadto higher resolution imaging, it would make the signal spread out in a wide range of magnetic field, leading to even worseSNR and unacceptable noise. Figure 3a and b present theprojections of the spin concentrations on the ZY and XYplanes, respectively. One can clearly observe that the signal isdistributed in an area of 8 × 4 mm and a thickness of 1.5 mm,consistent with the size of the separator used in the cell (Figure3c). Therefore, the unknown species should reside in theelectrolyte. To ensure this conclusion, the separators extractedfrom the coin cells were examined by ex situ EPR experiments(Figure S11), where the same signal was observed as well. The unknown signal can be originated from radicals ordissolved Cr3+/5+ ions in the electrolyte. Different radicals werereported to form from the reduction of the solvent moleculesby anodes.39-41 We compared the 'H and l3c NMR spectra ofthe electrolyte at 3.5 and 3.9 V (Figure S12), and no apparentvariations can be discerned. Besides, the generation of thesignal is unlikely to be associated with the anode process,otherwise it would not show a relevance to the phasetransition. The Cr contents in the separators at different statesof charge (SOCs) were determined by ICP-MS, and the resultsare summarized in Table S1. When it is charged to the capacityless than 150 mAh g,the Cr concentrations are lower thanthe detection limit. Above 150 mAh g, Cr can be detectedand the contents are elevated as the SOC rises, up to amaximum at 4.5 V, corresponding to 5.214 × 10- of Crdissolved from the cathode. Note that the actual dissolved Cramounts should be higher because some electrolyte is residualin the coin cells disassembled. The amount of EPR sample is proportional to the doubleintegral of the first derivative EPR spectrum, by which theamounts of Mn + ions dissolved from LiMn2O4 have beensuccessfully determined.42,43 Here, the dissolved Cr ratio isdetermined via dividing the doubly integrated EPR signal ofthe Cr ions by that of the pristine NaCrO2. As shown in Figure4, the order of magnitude of the dissolved Cr ratio by EPR is in Figure 4. Dissolved Cr ratio determined via dividing the doublyintegrated EPR signal of the Cr ions by that of the pristine NaCrOz.The data used in the calculation is from the EPR spectra in Figure 2c.Of note is that the electron spin (S) is different for Cr’ and Crt, sothe double integral is normalized by a factor of [S(S +1)]1/? beforethe Cr ratio is calculated. agreement with the result obtained by ICP-MS. Given that thedissolved Cr ratio is too little, we believe that the Cr ions arenot originated from the Cr migration in the bulk but from thesurface reconstruction, that is, a densification from layeredNaCrO, to rock-salt CrO. In previous research, Cr migrationwas proposed to occur via the charge disproportionation/comproportionation ofCr(3Cr*→2Cr++ Cr+), whichdepends on the presence of Cr++-Cr*+-Cr* triplets. Here,we suggest a disproportionation-dissolution mechanism for the surface reconstruction involved with the disproportiona-tion of Cr-Cr doublets (Cr+ Cr*→ Cr++ Cr+) andsubsequent Cr+ dissolution. The Cr+ dissolution will causethe reaction to proceed to the right, thereby generating moredivalents and leading to the surface densification. Another interesting finding is that the dissolved Cr ratio onlyincreases during the first charge to 4.5 V and keeps a constantlevel during the second charge, while it decreases quickly in thefirst and second discharge process. We propose that thelayered-to-rock-salt transformation is completely finished uponcharging to 4.5 V, such that no more Cr dissolution is observedin the second charge, and the Crions can be reduced on thecathode electrode during discharging, although why the solublemetal ions do not react with the Na anodes is still a mystery. Does Cr dissolution take place in other Cr-based cathodematerials? LiCrO, the lithium equivalent of NaCrO, has apoor electrochemical inactivity due to a layered-to-rock-salttransformation in the surface regions.4 Operando EPR ishence carried out on LiCrO, to examine the existence of theCrions. As shown in Figure S13, an analogous signal with g=1.976 and △Bp=0.4 mT arises at 4.2V, which confirms theoccurrence of dissolved Crions during the charge process ofLiCrOz. This finding highlights the importance of theinterfacial process in Cr-based cathode materials. In conclusion, the electrochemical processes of NaCrO,cycled within reversible (2.2-3.6 V) and irreversible (2.2-4.5V) regions are investigated by operando EPR. In the reversibleregion, an electronic delocalization/relocalization is suggestedto occur at low SOC accompanied by a rhombohedral-to-monoclinic phase transition. The electronic delocalizationremains even when charging to 4.5 V, while a new sharp signalarises along with the irreversible layered-to-rock-salt trans-formation above 3.75 V. Through EPR imaging, the unknownsignal is located in the electrolyte, which is then attributed tothe dissolved Cr’ions. We propose a different mechanism ofthe Cr disproportionation on the surface, which depends onthe presence of Cr+-Crdoublets and forms rock-salt CrOand soluble Crs+ ions. Operando EPR is a convenient tool to monitor in real timethe evolution of the electronic property upon charging anelectrode material. For the first time, trace amounts of Cr ionsdissolved from the cathode are detected due to the ultrahighsensitivity of EPR technique. This work contributes to theunderstanding of the electrochemical process of Cr-basedsystems, which has the potential to be the low-cost and high-performance cathode materials for next-generation batteries. ASSOCIATED CONTENT s Supporting Information The Supporting Information is available free of charge athttps://pubs.acs.org/doi/10.1021/acs.jpclett.0c03327. Experimental methods, digital photographs of theoperando cell, XRD pattern of NaCrO 2Na NMRand CW-EPRRspectra of NaCrO2, electrochemicalmeasurements, ex situ XRD experiments, ex situ 23NaNMR experiments, additional operando EPR spectra ofNaCrO, EPR spectra of the separators, H and 13csolution NMR spectra of the electrolyte, ICP-MS results,and operando EPR spectra of LiCrOz (PDF) Corresponding Author Bingwen Hu - Shanghai Key Laboratory of MagneticResonance, State Key Laboratory ofPrecision Spectroscopy,School of Physics and Electronic Science, East China NormalUniversity, Shanghai 200062, P.R. China;o orcid.org/0000-0003-0694-0178; Email: bwhu@phy.ecnu.edu.cn Authors Fushan Geng - Shanghai Key Laboratory of MagneticResonance, State Key Laboratory of Precision Spectroscopy,School of Physics and Electronic Science, East China NormalUniversity, Shanghai 200062, P.R. China Qi Yang - Shanghai Key Laboratory of Magnetic Resonance,State Key Laboratory of Precision Spectroscopy, School ofPhysics and Electronic Science, East China NormalUniversity, Shanghai 200062, P.R. China; @orcid.org/0000-0002-7528-8999 Chao Li - Shanghai Key Laboratory of Magnetic Resonance,State Key Laboratory of Precision Spectroscopy, School ofPhysics and Electronic Science, East China NormalUniversity, Shanghai 200062, P.R. China;@ orcid.org/0000-0002-0153-7825 Bei Hu - Shanghai Key Laboratory of Magnetic Resonance,State Key Laboratory of Precision Spectroscopy, School ofPhysics and Electronic Science, East China NormalUniversity, Shanghai 200062, P.R. China Chong Zhao - Shanghai Key Laboratory of MagneticResonance State Key Laboratory of Precision Spectroscopy,School of Physics and Electronic Science, East China NormalUniversity, Shanghai 200062, P.R. China Ming Shen - Shanghai Key Laboratory of MagneticResonance, State Key Laboratory of Precision Spectroscopy,School of Physics and Electronic Science, East China NormalUniversity, Shanghai 200062, P.R. China; D orcid.org/0000-0003-1343-2761 Complete contact information is available at: https://pubs.acs.org/10.1021/acs.jpclett.0c03327 Notes The authors declare no competing financial interest. ACKNOWLEDGMENTS This work was supported by the National Natural ScienceFoundation of China (21872055,21902049,21703068,21874045) and the Shanghai Sailing Program (19YF1413000). REFERENCES ( (1) V aalma, C.; Buchholz, D . ; W e il, M . ; P a sserini, S. A c o st a n dresource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018,3 (4), 1-11. ) ( (2) Delmas, C .; Carlier, D.; Guignard, M. The L ayered Oxides inLithium and S odium-Ion Batteries: A Solid-State Chemistry Approach. Adv. Energy Mater. 2020, 2001201. ) ( (3) Delmas, C. S odium and S o dium-Ion Batteries: 50 Y ears o fResearch. Adv. Energy Mater. 2018,8(17),1703137. ) (4) Liu, T.; Zhang, Y.; Jiang, Z.; Zeng, X.; Ji, J.; Li, Z.; Gao, X.; Sun,M.; Lin, Z.; Ling, M.; et al. Exploring competitive features ofstationary sodium ion batteries for electrochemical energy storage.Energy Environ. Sci. 2019, 12(5),1512-1533. ( (5) Komaba, S.; Takei, C.; N akayama, T.; O g ata, A . ; Y a buuchi, N. Electrochemical intercalation activity of layered NaCrO, vs. L iCrO. Electrochem. Commun. 2010, 12 ( 3),355-358. ) ( (6) Reed, J.; Ceder, G.; Van Der Ven, A. Layered-to-Spinel Phase T r a nsition in Li MnO. Electrochem. Solid-State Let t . 20 0 1, 4 ( 6 ) , A78. ) ( (7) Armstrong, A . R . ; B r uce, P . G. S y nthesis of layered LiMnOz asan e lectrode f or r echargeable l ithium b atteries. Nature 1 996,381(6582), 499-500. ) ( (8) C ho,J.; K i m, Y. J; Kim, T. J.; Park, B. Effect of AlOs-coated o- L iMnO cathodes prepared at various temperatures on the S5 degrees C cycling behavior. J. E lectrochem. S oc. 2 002, 1 49 ( 2 ), A127-A132. ) ( ( 9) B illaud, J.; Clement, R. J.; Armstrong, A. R.; Canales-Vazquez, J; Rozier, P.; Grey, C. P.; Bruce, P. G. B-NaMnOz: A High-PerformanceCathode f o r Sodium-Ion Batteries. J. Am. C h em. Soc. 2014, 136 (49), 17243-17248. ) (10) Chen, X.; Wang, Y.; Wiaderek, K.; Sang, X.; Borkiewicz, O.;Chapman, K.; LeBeau, J.; Lynn, J; Li, X. Super Charge Separationand High Voltage Phase in NaMnOz. Adv. Funct. Mater. 2018, 28(50),1805105. ( ( 11) Yabuuchi, N . ; K a jiyama, M.; Iw a tate, J.; Nishikawa, H.; H i tomi, S.; Okuyama, R . ; U s ui, R . ; Yamada, Y . ; K o m aba, S. P2- t ype Nay[Fe1/2Mn1/2]02 made from earth-abundant elements for recharge-able Na batteries. Nat. Mater. 2012, 11 (6), 512-517. ) ( (12) M u, L . ; Xu, S . ; Li, Y .; Hu, Y.; L i, H.; C hen, L.; H uang, X.Prototype Sodium-Ion Ba t teries Using an Ai r -Stable and Co/Ni-FreeO3-Layered M e tal Oxide Cathode. Adv. Mater. 2015, 27 (43), 6 9 28- 6933. ) ( (13) Hirayama, M.; Tomita, H.; Kubota, K.; Kanno, R. Structure andelectrode reactions o f layered r o cksalt L i FeO, nanoparticles forlithium battery cathode. J. Power Sources 2011, 196 (16), 6809-6814.(14) S ilvan, B.; Gonzalo, E.; D juandhi, L.; S h arma, N. ; Fauth, F.; Saurel, D. O n t h e d y namics of transition metal mi g ration and its impact on the performance of layered oxides for sodium-ion batteries:NaFeO, a s a case study. J. Mater. Chem. A 2018, 6(31), 15132- 15146. ) ( (15) X ia, X .; D ahn, J. R . N aCrO, is a Fundamentally Safe PositiveElectrode Material for Sodium-Ion Bat t eries with Liquid Electrolytes. E lectrochem. Solid-State Lett. 2012 , 15 ( 1 ),A1. ) (16) Wang, Y.; Li, W.; Hu, G.; Peng, Z.; Cao, Y.; Gao, H.; Du, K.;Goodenough, J. B. Electrochemical Performance of Large-GrainedNaCrO Cathode Materials for Na-Ion Batteries Synthesized byDecomposition of NazCr,O 2H,O. Chem. Mater. 2019, 31 (14),5214-5223. (17) Liang, L.; Zhang, W.; Denis, D. K.; Zhang, J.; Hou, L.; Liu, Y.;Yuan, C. Comparative investigations of high-rate NaCrO2 cathodestowards wide-temperature-tolerant pouch-type Na-ion batteries from- 15 to SSC: nanowires vs. bulk. . Mater. Chem. A 2019, 7 (19),11915-11927. (18) Yu, C.; Park, J.; Jung, H.; Chung, K.; Aurbach, D.; Sun, Y.;Myung, S.NaCrO, cathode for high-rate sodium-ion batteries. EnergyEnviron. Sci. 2015, 8 (7), 2019-2026. (19) Kubota, K.; Ikeuchi, I.;Nakayama, T.; Takei, C.; Yabuuchi, N.;Shiiba, H.; Nakayama, M.; Komaba, S. New Insight into StructuralEvolution in Layered NaCrO, during Electrochemical SodiumExtraction. J. Phys. Chem.C 2015, 119 (1), 166-175. (20) Bo, S.; Li, X.; Toumar, A. J.; Ceder, G. Layered-to-Rock-SaltTransformation in Desodiated NaCrOz (x 0.4). Chem. Mater. 2016,28(5),1419-1429. (21) Stoyanova, R.; Barra, A. L.; Yoncheva, M.; Zhecheva, E.;Shinova, E.; Tzvetkova, P.; Simova, S. High-Frequency ElectronParamagnetic Resonance Analysis of the Oxidation State and LocalStructure of Ni and Mn Ions in Ni,Mn-Codoped LiCoO. Inorg.Chem. 2010, 49 (4), 1932-1941. (22) Zhecheva, E.; Stoyanova, R.; Alcantara, R.; Lavela, P.; Tirado,J.L. EPR studies of Li deintercalation from LiCoMnO4 spinel-typeelectrode active material. J. Power Sources 2006, 159(2),1389-1394.(23) Kalapsazova, M.; Ivanova, S.; Kukeva, R.; Simova, S.; Wegner,S.; Zhecheva, E.; Stoyanova, R. Combined use of EPR and 2Na MASNMR spectroscopy for assessing the properties of the mixed cobalt-nickel-manganese layers of P3-NayCo1-2NiMn,O2. Phys. Chem.Chem. Phys. 2017, 19 (39), 27065-27073. (24) Li, C.; Shen, M.; Lou, X.; Hu, B. Unravel the Redox Couples of.v"/v Mixed Valent NaV(PO)0F14 Cathode by ParallelMode EPR and In-Situ/Ex-Situ NMR. J. Phys. Chem. C 2018, 122(48),27224-27232. ( (25) N iemoller, A.; Jakes, P .; Eichel, R.; Granwehr,J . In operandoEPR i n vestigation of redox mechanisms in LiCoO. Chem. Phys. Lett 2019,716,231-236. ) ( .(26) Niemoller, A.; J a kes, P . ; E u rich, S. ; Paulus, A.; Kungl, H.; Eichel, R.; Granwehr, J. Monitoring l ocal redox p rocesses in LiNiosMnisO4 b a ttery c athode m aterial by in operando E PRspectroscopy. J. Chem. P hys. 2018, 148 ( 1 ), 014705. ) ( (27) Tang, M.; Da l zini, A. ; Li , X.; Feng, X.; Ch i en, P.; Song, L.; Hu,Y. Operando E PR for S i multaneous Monitoring o f A n ionic andCationic R edox P r ocesses in Li-Rich Metal Ox i de Cat h odes. J. Phys.Chem. Lett . 2017,8(17),4009-4016. ) ( (28) W andt, J.; Marino, C.; G a steiger, H . A.; Jakes, P . ; Eichel, R .;Granwehr,J. O p erando electron paramagnetic resonance spectrosco-py - formation of m o ssy lithium on lithium anodes during charge- discharge c ycling. E nergy Environ. Sci. 2015, 8 (4), 1358-1367 . ) ( (29) W andt, J.; Jakes, P.; Granwehr, J.; Eichel, R.; Gasteiger, H. A.Quantitative and t ime-resolved detection o f l i thium p l ating ongraphite a nodes i n l i thium ion batteries. M a ter. Today 2018, 21 (3), 231-240. ) ( ( 30) Wang, Q. ; Zh e ng, J.; Walter, E. ; Pan, H. ; Lv, D. ; Zu o , P.; Ch e n, H.; Deng Z. D.; L i aw, B. Y.; Yu, X.; et al. Direct Observation of Sulfur Radicals as R eaction Media in Lithium Sulfur Batteries. J.Electrochem.Soc.2015,162(3), A474-A478. ) ( (31) Wandt, J; Jakes, P.; G ranwehr, J.; Gasteiger, H. A.; Eichel, R. Singlet Oxygen F o rmation du r ing the Charging Process of an Apr o tic Lithium-Oxygen Battery. Angew. Chem. 2016, 1 28(24),7006-7009 . ) ( (32) Sathiya, M.; Leriche, J. B .; S alager, E.; Gourier, D.; Tarascon, J.M.; V ezin, H . E lectron paramagnetic resonance im a ging for real-timemonitoring of Li-ion batteries. Nat. Commun. 2015, 6( 1 ), 1-7. ) ( (33) Niemoller, A.; Jakes, P.; Eiche l , R.; Granwehr, J. EPR Imagingof Metallic Lithium and its Application t o D e ndrite Lo c alisation inBattery S eparators. S ci. Rep. 2018, 8 ( 1), 14331-7. ) ( (34) D utoit, C. E . ; Tang, M.; Go u rier, D.; Tar a scon, J. M . ; Vezi n ,H.; Salager, E. In Situ Electron Paramagnetic Resonance C o rrelated Spectroscopy and I maging: A T o ol for Li t hium-Ion Ba t teries t o Investigate Metalli c Lithium Sub-Micrometric Structures Created by Plating and S tripping. ChemRxiv, ver. 1; 2 020; DOI: 1 0.26434/chemrxiv.12939776.v1. ) ( (35) Z heng, L.; B e nnett, J. C.; O brovac, M. N. Sta b ilizing NaCrOz by S odium S i te Doping with Calcium. J. E l ectrochem. Soc. 2019, 1 66 (10), A2058-A2064. ) ( ( 36) L i ,W . ; Wang, Y.; Hu , G.; Pen g , Z.; Cao , Y.; Zeng , Y.; Du, K .Ti-doped NaCrO as c athode mat e rials for sodium-ion batteries w i th excellent long cycle life.J. Alloys Compd. 2 019, 7 7 9, 147-155. ) ( ( 37) Zhou, Y.; D i ng, J. ; Nam, K. ; Yu , X. ; Bak, S. ; H u , E.; Li u , J.; Bai,J; L i, H.; F u, Z.; e t a l. Phase t ransition behavior of NaCrO, duringsodium extraction studied by synchrotron-based X-ray diffraction andabsorption spectroscopy. J. Mater. Chem. A 2013, 1 ( 37), 11130. ) ( (38) M enetrier, M.; S aadoune, I .; Levasseur, S.; Delmas, C. Theinsulator-metal transition upo n lithium deintercalati o n fr o m LiCoO: electronic properties and L i-7 N MR study. J. Mater. Ch e m. 199 9 , 9 (5), 1 135-1140. ) ( (39) Jin, Y. ; Kneusels , N . H. ; Magusin, P. C. M. M.; Kim, G .; Castillo-Martinez, E.; Marbella, L . E.; K erber, R . N.; Howe, D. J; Paul, S.; Liu, T.; e t a l. I dentifying t he S tructural B asis f or t h e Increased S tability of the Solid Electrolyte Interphase Formed o n Silicon with the Additive Fluoroethylene Carbonate. J . Am. Chem. Soc.2017, 139(42), 1 4992-15 0 04. ) ( (40) Jin, Y.; Kneusels, N . H.; Grey, C . P. NMR S tudy of t heDegradation P roducts of Ethylene Carbonate in Silicon-Lithium IonBatteries.J. Phys . Chem. Lett. 2019 , 10 (20) , 6345-6350. ) (41) Rinkel, B. L. D.; Hall, D. S.; Temprano, I.; Grey, C. P.Electrolyte Oxidation Pathways in Lithium-Ion Batteries. J. Am. Chem.Soc. 2020, 142(35),15058-15074. ( (42) Shilina, Y.; Ziv, B.; Meir, A.; Banerjee, A.; Ruthstein, S.; Luski, S.; Aurbach, D.; Halalay, I . C . Combined E lectron Paramagnetic Resonance a n d A t omic Ab s orption Spe c troscopy/Inductively Coupled P l asma A n alysis A s D i agnostics f o r S o luble Manganese Species f rom Mn-Based Positive El e ctrode Materials in Li - ion Ce l ls.Anal. Chem. 2016, 88 ( 8), 4440-4447. ) ( (43) B a nerjee, A. ; Sh i lina, Y.; Ziv, B. ; Zi e gelbauer, J. M.; Lus k i , S.; Aurbach, D.; Halalay, I. C. On the Oxidation State of Manganese Ionsin Li-Ion B attery Electrolyte Solutions. J. Am. C h em. Soc. 20 1 7, 139 (5),1738-1741. ) ( ( 44) Lyu, Y . ; B e n, L . ; Sun, Y.; T a ng, D.; Xu, K. ; Gu, L. ; Xi a o, R. ; Li , H.; Chen, L.; H uang, X. Atomic insight in t o electrochemical inactivityof lithium chromate (LiCrO2): Irreversible migration of chromium into lithium l ayers i n s urface r egions. J . Power Sources 2015, 2 7 3,1218-1225. ) https://dx.doi.org/acs.jpclett.cCS Publications@ XXXX American Chemical Society. Phys. Chem. Lett. ttps://dx.doi.org/acs.jpclett.c. Phys. Chem. Lett. 布鲁克的高性能电子顺磁共振(EPR)产品组合提供了一种非破坏性的分析技术,可直接检测顺磁性物质(含自由基和过渡金属离子)。自由基和过渡金属离子可以在固体、液体、气体或细胞和体内进行鉴定和定量。通过连续波和脉冲EPR 技术可以获得化学结构和分子间的相互作用等。 在布鲁克的EPR 波谱仪系列中,ELEXSYS-E580 是一款傅立叶变换(FT)/连续波(CW)系统,为多频和多共振EPR奠定了基础。作为一台高度通用的仪器,E580 可以进行当今所有的脉冲/连续波实验,并为未来的挑战做好准备。 E580 采用中频设计,便于从标准的X 波段升级到L、S、Q 和W 波段;其独有的SpecJet-III 数字采集器和VAMP-III 视频放大器可提供高达1 GHz 的检测带宽;SpinJet AWG(任意波形发生器)开启了形状脉冲的新领域,显著提升了灵敏度和选择性。 2019年,华东师范大学物理与电子科学学院研究员胡炳文老师采购了布鲁克E580 连续波/脉冲电子顺磁共振波谱仪(包括L-波段,X-波段和Q-波段,以及成像系统)。E580 凭借其优越的检测能力,为多种波谱、成像实验提供了稳健的技术支持。 近期,胡老师利用布鲁克E580 电子顺磁共振波谱仪,在国内首次利用EPR 成像技术进行了钠离子电池性能研究,并发表重要论文。我们诚邀您阅读这篇学术佳作。论文摘要 NaCrO2 因其低成本、高安全性和高功率的特点,成为钠离子电池正极的备选材料之一。在投入实际应用前,有必要进一步理解其在循环过程中的电子结构和相变特性。 本研究中,我们采用原位EPR 对NaCrO2 在2.2 - 3.6 V 和2.2 - 4.5 V 电压条件之间循环切换时电子结构的演变进行监测。我们发现在充电初期NaCrO2 脱钠以后产生了电子离域效应,这或许是其具备卓越倍率性能的原因。 此外,通过原位EPR 和EPR 成像,我们发现在3.8 V 条件下,电解液中出现了与不可逆相变相关的EPR 信号,这是由于表面重构生成了Cr5+ 离子、而Cr5+ 离子溶解在电解液并存在于较厚的电池隔膜当中。这些研究结果可以帮助研究者更好地设计和改性铬基正极材料。 作者简介 胡炳文,现任华东师范大学物理与电子科学学院研究员,法国里尔第一大学博士,国基优青项目获得者。胡老师的主要研究领域为磁共振方法及其在电池领域中的应用,他开发了SHANGHAI、SHA+、RFDF-XY8-4-1和COM-IV等固体核磁共振脉冲序列,开发了电池体系的in situ NMR 和in situ EPR与EPR 成像方法,应用于锂/钠电池体系。
关闭-
1/6
-
2/6
还剩4页未读,是否继续阅读?
继续免费阅读全文产品配置单
布鲁克磁共振事业部(Bruker Magnetic Resonance)为您提供《NaCrO2中循环过程的电子结构和相变特性检测方案(顺磁共振波谱)》,该方案主要用于电子元器件产品中循环过程的电子结构和相变特性检测,参考标准《暂无》,《NaCrO2中循环过程的电子结构和相变特性检测方案(顺磁共振波谱)》用到的仪器有布鲁克 ELEXSYS II 系列电子顺磁共振(EPR)波谱仪.。
我要纠错
推荐专场
电子自旋(顺磁)共振波谱仪(ESR)
更多相关方案






 咨询
咨询




