方案详情文
智能文字提取功能测试中
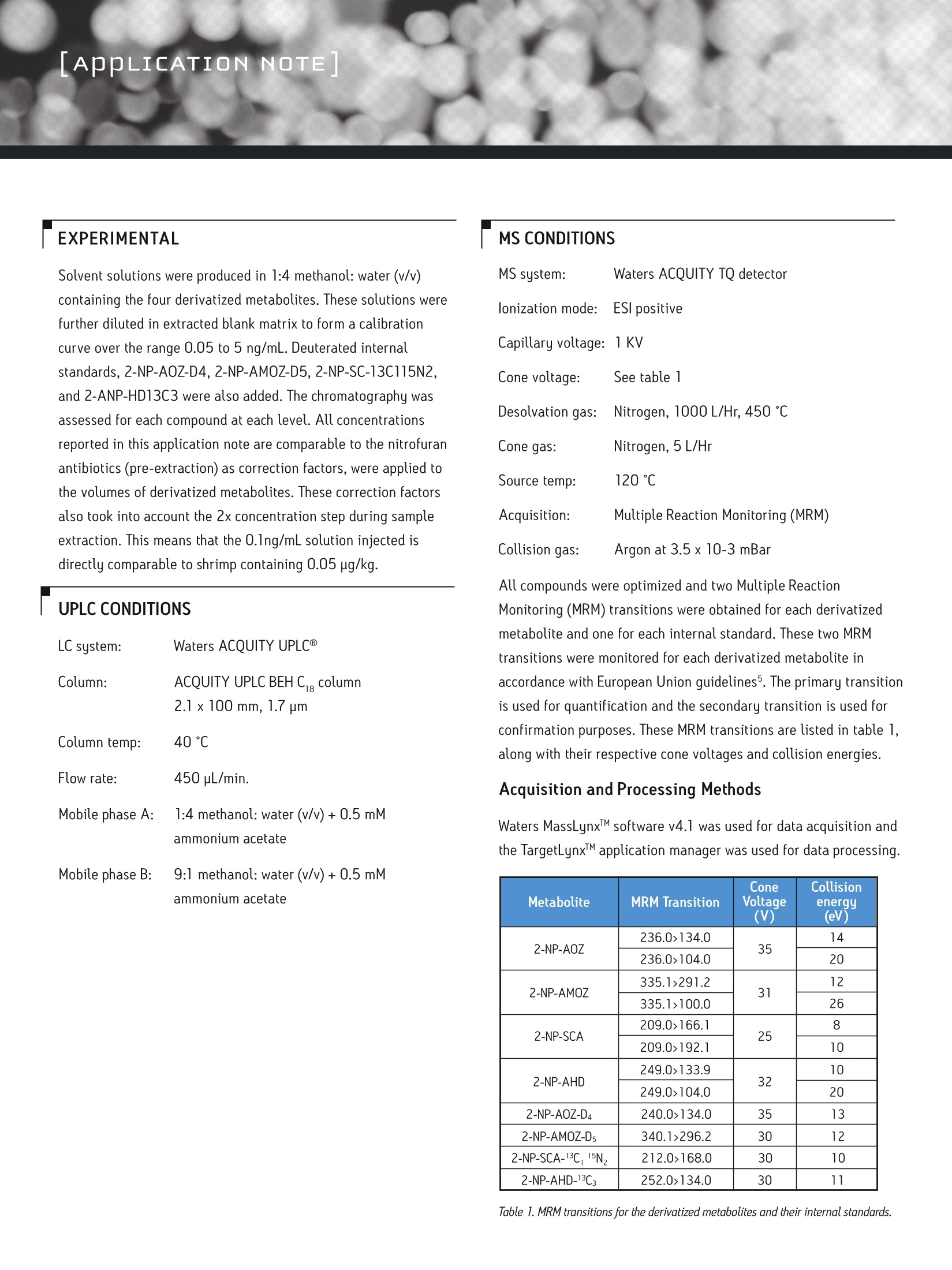
O2007 Waters Corporation. Produced in the U.S.A.July 2007 720002299EN RB-PDF APPLICATION NOTEWatersTHE SCIENCE OF WHAT'S POSSIBLE, APPLICATION OF ACQUITY TQD FOR THE ANALYSIS OF NITROFURAN VETERINARYDRUG RESIDUES IN SHRIMP James Morphet and Peter Hancock, Waters Corporation,Manchester, UK INTRODUCTION This application note describes the transfer of an existing UPLCmethod to the tandem quadrupole mass spectrometer, Waters@ACQUITY@ TQD (Figure 1). Standard solutions spiked into blankmatrix extract were used to assess chromatography as there willnot be changes to the extraction of nitrofuran metabolites fromedible tissues. The four drugs furazolidone, furaltadone, nitrofurazone, andnitrofurantoin are veterinary drugs that belong to the family ofnitrofuran antibiotics and are banned in meat destined for humanconsumption?. These nitrofurans are readily metabolized inanimals to undetectable levels, metabolizing to 3-amino-2-ox-azolidinone (AOZ), 3-amino-5-morpholinomethyl-2-oxazolidinone(AMOZ), semicarbazide (SC) and 1-aminohydantoin (AHD),respectively. The structures of the nitrofuran antibiotics and theirfree metabolites are shown in figure 2. The metabolites are morepersistent in edible tissues and can be used to detect use of theparent drug. Proportions of these metabolites exist as proteinadducts and the established method of analysis contains acidhydrolysis and derivatization steps prior to extraction3. The European Union (EU) has banned the use of these substances,documenting them in Council Regulation 2377/90, annex IV. Figure 1. ACQUITY TQD featuring the TQ detector. A Minimum Required Performance Limit (MRPL) of 1ug/kg hasbeen set to which laboratories should be able to detect andconfirm these compounds. Concerns over nitrofurans are relatedto potential carcinogenicity (causing cancer) and genotoxicity(causing damage to genetic material in cells) of the drugsand their metabolites as well as the ability to cause allergicreactions4. The introduction of the ACQUITY TQD (Figure1) allows scientiststo perform nitrofuran metabolite analysis while harnessing allthe benefits that this new instrument brings to the laboratory.The IntelliStartM technology in this instrument is designed toreduce the burden of complicated operation, time-intensivetroubleshooting, and upkeep. Its small footprint will give anylaboratory an advantage as this powerful tool removes the needfor larger instrumentation. This note describes an extended nitrofuran method, for fourresidues and their associated internal standards, which exceedsthe requirements for analysis of these regulated compounds. AppLICATION NOTE Solvent solutions were produced in 1:4 methanol: water (v/v)containing the four derivatized metabolites. These solutions werefurther diluted in extracted blank matrix to form a calibrationcurve over the range 0.05 to 5 ng/mL. Deuterated internalstandards,2-NP-AOZ-D4,2-NP-AMOZ-D5, 2-NP-SC-13C115N2,and 2-ANP-HD13C3 were also added. The chromatography wasassessed for each compound at each level. All concentrationsreported in this application note are comparable to the nitrofuranantibiotics (pre-extraction) as correction factors, were applied tothe volumes of derivatized metabolites. These correction factorsalso took into account the 2x concentration step during sampleextraction. This means that the 0.1ng/mL solution injected isdirectly comparable to shrimp containing 0.05 ug/kg. UPLC CONDITIONS LC system: Waters ACQUITY UPLC Column: ACQUITY UPLC BEH Cig column 2.1 x 100 mm, 1.7 um Column temp: 40°℃ Flow rate: 450 uL/min. Mobile phase A: 1:4 methanol: water (v/v)+0.5 mM ammonium acetate Mobile phase B: 9:1 methanol: water (v/v)+0.5 mM ammonium acetate MS CONDITIONS MS system: Waters ACQUITY TQ detector lonization mode: ESl positive Capillary voltage: 1 KV Cone voltage: See table 1 Desolvation gas: Nitrogen, 1000 L/Hr, 450 ℃ Cone gas: Nitrogen, 5 L/Hr Source temp: 120℃ Acquisition: Multiple Reaction Monitoring (MRM) Collision gas: Argon at 3.5x10-3 mBar All compounds were optimized and two Multiple ReactionMonitoring (MRM) transitions were obtained for each derivatizedmetabolite and one for each internal standard. These two MRMtransitions were monitored for each derivatized metabolite inaccordance with European Union guidelines5. The primary transitionis used for quantification and the secondary transition is used forconfirmation purposes. These MRM transitions are listed in table 1,along with their respective cone voltages and collision energies. Acquisition and Processing Methods Waters MassLynxIM software v4.1 was used for data acquisition andthe TargetLynxM application manager was used for data processing. Metabolite MRM Transition ConeVoltage () Collision energy(eV) 2-NP-AOZ 236.0>134.0 35 14 236.0>104.0 20 2-NP-AMOZ 335.1>291.2 31 12 335.1>100.0 26 2-NP-SCA 209.0>166.1 25 8 209.0>192.1 10 2-NP-AHD 249.0>133.9 32 10 249.0>104.0 20 2-NP-AOZ-DA 240.0>134.0 35 13 2-NP-AMOZ-D5 340.1>296.2 30 12 2-NP-SCA-13C,15N, 212.0>168.0 30 10 2-NP-AHD-13C: 252.0>134.0 30 11 Table 1. MRM transitions for the derivatized metabolites and their internal standards. RESULTS AND DISCUSSION All nitrofurans were separated successfully. Figure 3 shows thetotal ion chromatogram (TIC) for all derivatized metabolites.Only one time window was required for the eight compoundsbeing analyzed. A dwell time of 0.025 seconds was used givingapproximately 14 data points across each peak. Figure 3. TIC showing the four nitrofuran metabolites in shrimp matrix at1 ng/mL(equivalent to 2ug/kg in shrimp). The limit of detection achieved for all components of the deriva-tized metabolite mixture was less than 0.05 ng/mL (equivalent to0.025 ug/kg in shrimp). This is forty times below the EU minimumrequired performance limit (MPRL) guideline. The 0.05 ng/mLmatrix-matched standard for 2-NP-AMOZ is illustrated in Figure 4. A calibration curve was spiked over the range of 0.05-5 ng/mLwhich gave a good linear response (r2=0.9972). Good peak shapewas achieved for both analyte and internal standard. The lowestquantifiable level achieved by the TQD in this analysis was 0.05ng/mL. This level gave an acceptable S:N of 5:1 or greater for allfour nitrofuran metabolites in shrimp with 50pL injection volume.This level is twenty times lower than the MPRL set by the EU. Figure 4. TargetLynx screen shot showing response for 2-NP-AMOZ at 0.05 ng/mL(0.025pug/kg in shrimp). A run of 50 samples in matrix at a concentration of 5 ng/mL wasinjected. This was used to assess robustness of ACQUITY TQDresponse over a four hour run. Injections of 10 uL were performedas a limited amount of sample was present. Figure 5 shows a plotof injection number vs. response ratio (analyte peak area/internalstandard peak area). There is no noticeable change in response forany compound over the course of the run. Figure 5. Plot showing peak area over 50 injections in shrimp. Extracts were injectedat a concentration of 5 ng/mL. The percent relative standard deviation (%RSD) isshown in the graph header. APPLICATION NOTE Figure 6 illustrates the robustness of the ion ratios over the same50 injections. This plot shows that there is no significant change inion ratio for any compound over the course of the run. A stable ionratio is fundamental when confirming the identity of compounds. Figure 6. Plot showing peak area over 50 injections in shrimp. Extracts were injectedat a concentration of 5 ng/mL. All injections lie within the ±20% boundary requiredfor confirmation with the average difference shown in the header of the graph. CONCLUSION The established UPLC method for the determination of nitrofuranveterinary drug residues, which are hazardous to human health,has been successfully transferred to ACQUITY TQD. The ACQUITY TQD provides sensitivity that allows quantifiabledetection at twenty times below the EU minimum required perfor-mance limit (MPRL) guideline for the four nitrofuran metabolites. Robustness of the ACQUITY TQD was proven with a 50 injectionsequence that did not show a major change (<20%) in response ratio with relative standard deviations being 8.3% or less for allcompounds. Confirmation was achieved using a secondary MRM transitionover a 50 injection sequence, where the variance was 11% orless in all cases showing it to be robust. This multiple nitrofuran method obsoletes the use of severalsingle nitrofuran methods where repeat analysis is required. The method allows the determination of multiple contaminantsper sample which enables a complete picture to be obtained ofexposure to these compounds from the human diet. The benefits of UPLC for a revenue conscious laboratory areshown with increased speed, while further reducing solventusage and therefore the costs of solvents and solvent disposal. ACKNOWLEDGEMENTS The authors would like to thank Richard Fussell and MichaelDickinson at the Central Science Laboratory (CSL), Sand Hutton,York, UK for kindly supplying the sample extracts that wereanalyzed in this project. REFERENCES 1. Analysis of Nitrofuran Veterinary Drug Residues using ACQUITY UPLCand Quattro PremierT" XE, Waters Application Note No. 720001951EN 2. CCommission Regulation (EC) 1442/95, Official J. European Communities,No.L143/26. 3. Analytica Chimica Acta, 483:91-98,2003. 4. Canadian Food Inspection Agency http://www.inspection.gc.ca 5. Commission Directive 2002/657/EC, Official J. European Communities,No.L221/8. THE SCIENCE OF WHAT'S POSSIBLE. Waters,ACQUITY, UPLC, and ACQUITY UPLC are registeredtrademarks of Waters Corporation.IntelliStart, MassLynx,TargetLynx,Quattro Premier, and The Science of What’sPossible are trademarks of Waters coprporation. All othertrademarks are the property of their respective owners. Waters Corporation 34 Maple StreetMilford, MA 01757 U.S.AT:15084782000 F: 1 508 872 1990
关闭-
1/4

-
2/4
还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
苏州市莱顿科学仪器有限公司为您提供《硝基呋喃中代谢物检测方案(液相色谱仪)》,该方案主要用于化工原料中代谢物检测,参考标准《暂无》,《硝基呋喃中代谢物检测方案(液相色谱仪)》用到的仪器有Waters2695(Alliance系统)液相色谱仪。
我要纠错
推荐专场
相关方案






 咨询
咨询






