方案详情文
智能文字提取功能测试中
02 04 Pharmaceutical analvsis Quality control of pharmaceuticals QMetrohm MetrohmLmmm is the global market leader in titration offers a complete portfolio for NIR and Raman analysis, in addition to all of the methods of ion analysis - titration, voltammetry, and ion chromatography is a Swiss company and manufactures exclusively in Switzerland grants a 3-year instrument warranty and a 10-year warranty on chemical suppressors for anion chromatography · provides you with unparalleled application expertise ·offers you more than 1800 applications free of charge ·supports you with dependable on-site service worldwide is not listed on the stock exchange, but is owned by a foundation · takes a sustainable approach to corporate management, putting the interests of customers and employees ahead of maximizing profit Metrohm - customized analysis for thepharmaceutical industry The high standards of approval authorities Authorities around the globe hold the pharmaceuticalindustry to very high standards of drug quality and safety.The standards are documented in official collections ofrecognized pharmaceutical rules in pharmacopoeias.They provide a legal consumer protection framework forensuring that drugs are used safely. Measurement andtesting procedures used in the context of drug testingidentify drugs and determine whether they can bereleased. Reliable instruments and methods are required to guar-antee these strict quality and safety standards. You can count on our support As a leading manufacturer of instruments for chemicalanalysis, we are well aware of the challenges you face.For this reason, Metrohm offers you not only the mostadvanced instruments, but also complete solutions foranalytical studies. Your partners at Metrohm arecompe-tent specialists who develop customized applications andoffer competent support in every aspect of regulatorycompliance. Discover the solutions Metrohm offers the pharmaceuti-cal industry and you in particular for ensuring the qualityand safety of your products. Chemical pharmaceutical analysis The history of pharmacology The search for medicines is nearly as old as humanityitself. Documentary evidence exists to show that activepharmaceutical ingredients from plant, mineral, and ani-mal sources were already being used for medicinal pur-r-poses by the earliest advanced civilizations (China, India,Mesopotamia and Egypt). Systematic descriptions ofmedicines have been handed down to us from Greekantiquity (Hippocrates, Theophrastus) and from theRoman Empire (Dioscorides,Galen). This knowledge wasadopted and further developed by Arab scholars (e.g.,Avicenna). This body of knowledge served for a long timeas an important basis for pharmacology. It was not untilthe 16th century that the science began its departurefrom the models passed down from antiquity. A typicalrepresentative of the new direction was Paracelsus,who- in 1537 - coined the famous phrase: 《The dose makes.the poison》 (《dosis sola facit venenum》). The path to organic synthetic drugs The significance of the advances that came with theemergence of organic chemistry at the dawn of the 19thcentury cannot be overstated. Although drug therapiesup to that point had been limited to naturally occurringsubstances and inorganic chemicals, this changed withthe targeted production of organic synthetic drugs basedon substances isolated from medicinal plants. Within avery short period, an unprecedented advance in pharma-ceutical synthesis led to a vast number of synthesizedactive pharmaceutical ingredients. Researchers had final-ly come to understand the relationship between theaction of these substances and their chemical structures. Determination of active ingredients, excipients,and impurities Pharmaceutical analysis provides information on theidentity, purity, content and stability of starting materials,excipients, and active pharmaceutical ingredients (API). Adistinction is made between analysis of the pure activepharmaceutical ingredients used to cure, alleviate, pre-vent, or identify illnesses and diseases (active ingredientanalysis) and analysis of drug products (drug productanalysis). Drug products come in various forms (oint-ments, tinctures,pills, lotions, suppositories, infusions,drops, etc.) and consist of the pharmaceutically activesubstance and at least one pharmaceutical excipient.Impurities are mainly introduced during the synthesis ofthe active ingredient, and are usually monitored accord-ing to both the directives of the ICH (InternationalConference on Harmonisation of Technical Requirementsfor Registration of Pharmaceuticals for Human Use) andthe pharmacopoeias. Pharmacopoeias and drug safety According to the World Health Organization (WHO),specifications and test methods for commonly usedactive ingredients and excipients are outlined in detail inmonographs contained in the national pharmacopoeiasof more than 38 countries. These include the UnitedStates Pharmacopeia (USP), the European Pharmacopoeia(Ph.Eur.), derived from a harmonization of the requlationsof a number of individual states, and the JapanesePharmacopoeia (JP), to name just a few examples. Thepharmacopoeias are official compendia containing statu-tory requirements pertaining to identity, content, quality,purity, packaging, storage, and labeling of active phar-maceutical ingredients and other products used fortherapeutic purposes. They are essential for anyone seek-ing to produce, test, or market medicinal products Test methods, tests, and USP-NF monographs Applications in accordance with pharmacopoeiasMetrohm is your qualified partner for all chemical-phar-maceutical analysis issues and for analytical methodsvalidation. In addition to their compliance with officialdirectives, Metrohm instruments and applications complywith many of the quality control and product approvaltest methods cited in pharmacopoeias Harmonization efforts Based on the harmonization effortssof thePharmacopoeial Discussion Group (PDG), this brochurerelates primarily to selected test methods and mono-graphs of the USP as representative of the pharma-copoias not mentioned here. The National Formulary(NF) is the official compendium of standards for excipi-ents and plant-based drugs. Structure of the USP-NF Four chapters form the backbone of the USP-NF. Onechapter provides the analytical tools of the pharma-copoeia with a detailed description of test methods andtests. Test methods numbered from <1> to <1000> aremandatory, whereas the procedures with numbers above<1000> are recommended. The most comprehensive chapter of the USP-NF containsa list of the USP monographs, listed alphabeticallyaccording to active pharmaceutical ingredient, whichprovide detailed descriptions of test methods, tests,requirements, and storage conditions. The scope of theNF monographs is an order of magnitude smaller and iscontained in a separate chapter. Another chapter definesthe reagents, indicators, and solutions to use. Test methods referenced in USP-NF monographs Application/parameters USP monograph Citation frequency of the test method Test methods Page pH value USP<791> In approx. 1400 USP monographs pH value measurement 6 In approx. 250 NF monographs Conductivity USP<645> Ultrapure water (pharma) Conductivity measurement 6 Various APIsVarious excipients USP<541> In approx. 250 USP monographsIn approx. 130 NF monographs Titration 7-11 Water content USP<921> MethodI In approx. 630 USP monographs In approx. 110 NF monographs Karl Fischer titration 12-13 Various APIs USP<621> In approx. 58 USP and 13 NF monographs 18-21 USP<1065> Ion chromatography Various excipientsAmino acids In 3 NF monographsIn 5 USP monographs USP<1052>, MethodI Various APIs Thiomersal USP<801> USP<341> In 8 USP monographs Various antimicrobial agent determinations Polarography 22-23 23 Heavy metals USP<232>, <233> In approx. 780 USP and 230 NF monographs Various parameters USP<1119> Various Near-infrared spectroscopy 26-28 Various process parameters Process-dependent Various Process analysis 29-33 electroactive pharmaceuticals using electrochemistry(page 24). The brochure concludes with a final chapteron the comprehensive services provided by Metrohm. Quality Service on pages 34 and 35. In addition to the above-mentioned methods, this bro-)-chure contains chapters on automated sample prepara-tion (pages 14-15), determination of oxidation stabilityin ointments and creams (pages 16-17), and analysis of Water for pharmaceutical use (water for injection) pH value The 867 pH Module provides everything you need tomeasure the pH value according to USP<791>: it meetsthe requirements of FDA Requlation 21 CFR Part 11thanks to either 900 Touch Control or tiamo full soft-ware. An electrode test can be performed in conjunctionwith tiamo or 900 Touch Control. Conductivity andpH value can be measured in the same vessel when the856 Conductivity Module is combined with the 867 pHModule. Conductivity Particularly strict regulations apply to the measurementof the conductivity of water for pharmaceutical use(water for injection) in accordance with USP<645>. Inaddition to the highest level of precision, the test mustfulfill all of the requirements of U.S. FDA Requlation21 CFR Part 11. Compliance is assured using the856 Conductivity Module in combination with the900 Touch Control or tiamo full. This measuring cell was developed especially for mea-surements in water with very low conductivity. Therobust stainless steel construction is easy to clean andideal suited for conductivity values < 300 pS/cm, andthus for measuring waters for pharmaceutical use. Application know-how from the experts Because of their simplicity and accuracy, titration meth-ods are used for a large proportion of the content deter-minations described in the monographs, for example inaccordance with USP<541>. Taking into account thelatest methodological findings, Metrohm has developedhundreds of titration methods with Metrosensors basedontheU.S. Pharmacopeia (USP)and EuropeanPharmacopoeia (Ph. Eur.). Some older USP methods are still based on high sampleweights with a titrant consumption of up to 50 mL.Based on Ph. Eur., Metrohm has reduced the sampleweights considerably, reducing titrant consumption to atmost 10 mL. All the methods were developed to enable you to adoptthem as SOPs (Standard Operating Procedures) in yourtitration laboratory. Photometric titrations Photometric EDTA titrations (chelatometry/complexometry) Characteristic fat and oil values Titrando- the smart titrator withcomprehensive security Thanks to its modular design, the Titrando System can beoptimally adapted to any application. Both as a stand-alone titrator and in combination with the 900 TouchControl or tiamo, it is in compliance with FDA Regulation21 CFR Part 11. Certified and smart dosing elements Metrohm dosing elements set new standards in reliabili-ty. An inconspicuous data chip contains all the data thatthe Titrando needs to perform titrations, i.e., serial num-ber, cylinder number, type of reagent, titer, last titerdetermination, shelf-life data, and much more. But that'snot all - the Titrando compares the data it has obtainedwith that of the selected method and issues an errormessage if they do not agree. iTrodes - intelligent electrodes The electrode is the most important part of any titrationsystem. The Titrando with iConnect and iTrodes quaran-tees complete traceability of the analytic result to everyparticipating component in the analysis. The chip inte-grated in the electrode head enables the storage ofimportant sensor data such as article number, serialnumber, calibration data, calibration history, and calibra-tion interval. All of the sensor data is uploaded auto-matically when the sensor is connected to the Titrando.This provides added security because the user is informedif the electrode type does not match the type specified inthe method. STAT titration with tandem dosing The determination of enzyme activity (lipase, trypsin,etc.) or the release of active ingredients from antacidtablets requires a titrator that rapidly adjusts to a presetpH value and keeps it constant over a long period.Tandem dosing prevents dosing interruptions whenburets are refilled during titration - a second buretteimmediately takes over dosing. This means that rapid andconsumption-intensive reactions can be monitored inreal time. Flexibility -from individual instruments to fullyautomated systems Increasing numbers of samples, time-consuming samplepreparation, and unattended overnight operation are allgood reasons for using sample changers. When com-bined with the 814 USB Sample Processor, the 815Robotic USB Sample Processor XL, and the 898 XYZSample Changer, the Titrando offers a high degree ofautomation at a low investment cost. The 855 RoboticSample Processor combines a first-rate Robotic SampleProcessor with a Titrando and takes up minimal space. Sample preparation at the press of a button Metrohm instruments not only handle analysis itself, butas demonstrated by the 815 Robotic SoliPrep (seepages 14 and 15), also perform the most frequent sam-ple preparation steps. ·Homogenization The Polytron PT 1300 D made by Kinematica ensuresreproducible particle size reduction and homogeniza-tion of all samples. · Filtration Commercially available syringe filters with standardLuer connectors can be used directly with the RoboticSoliprep. · Liquid Handling From dilution series to sample filling in sealed vessels,there are no limits to Liquid Handling with Metrohm. Inepouction HtYsionas tiamo tiamo -titration and more tiamo is the leader in control and database software,not only for titrators and dosing devices, but also fortotal laboratory automation that includes the client/server system. The name tiamo stands for "titration andmore" - tiamo can do more thannjust titrate.tiamo is a titration network (NTDS= NetworkedTitration Data System). ·tiamo guarantees data security Whether you need to satisfy GMP or GLP rules, regula-tions regarding the security of electronic data, ortraceability requirement for results in accordance withthe FDA's Requlation 21 CFR Part 11, tiamo has beendeveloped from the ground up to comply with all ofthese requirements and is setting new standards. ·Signatures You can add digital signatures to determinations.tiamo offers two levels of signatures. The databecomes write-protected as soon as the determinationis assigned a level 2 signature. ·User administration Assign the access rights of each user according to yourcompany’s in-house security policy. tiamo sets nolimits and impresses with its unique flexibility. ·Complete audit trail Every user-performed action is automatically stored inthe audit trail. Audit data is available at the press of abutton. 。Traceability Play it safe and entrust your titration work only to thetraceability of Metrohm software and hardware. Everydata record contains all the raw data necessary toensure traceability. ·Data export tiamo offers you a wide selection of export formats,including XML. Your data are available to all conceiv- able Office applications, databases, and long-termarchiving programs. ·Reprocessing a determination Incorrect sample size? No problem. With the repro-cessing function you do not have to worry about yourdocumentation. tiamo automaticallyy records allchanges, thus providinga comprehensive overview. ·Report generator The report generator gives you full control over thedesign of your analytical reports. Modify one of themany report templates to suit your needs, and conve-niently send the report by e-mail. · Data security tiamo helps you ensure data security. When you acti-vate the automatic backup function, tiamo will thenautomatically make complete copies of all determina-tions and confiqurations. ·Client/server functionality In the simplest case you install the databases on yourlocal measurement computer. tiamo grows alongwith your requirements. You can configure tiamolater as a client/server whenever data security andcentral data management make this a requirement. ·Plug and play Our modern USB devices ensure that all connecteddevices appear in the devices window. Monitoringoptions rule out the use of system components thatare wrong or have expired. ·Parallel titration tiamo and Titrando make a powerful team. Paralleltitrations - if necessary, carried out by different users- increase efficiencv. Automatic nonaqueous titration with the MATi 03 Enormous time savings and results that are more accu-rate and precise are the crucial benefits of automation.The MATi 03 (Metrohm Automated Titration) is speciallydesigned for nonaqueous titrations in the pharmaceuti-cal industry - both potentiometric and photometric. Series of up to 59 samples can be accommodated on the815 Robotic USB Sample Processor XL. tiamo is respon-sible for all of the controls. The system is noteworthy forits high resistance to organic solvents. The MATi 03 with 815 Robotic USB Sample Processor XL (left) and 907 Titrando (center) Maximum precision Titrations using color indicators are still widely used inpharmacopoeiasS(chelatometry/complexometry), butwhen performed manually, the results depend, quite lit-erally, on the eye of the beholder. The new Optrodemakes it possible to replace this subjective determinationof the equivalence point with an objective process that iscompletely independent of the human eye. Its eightwavelengths, ranging from 470 to 660 nm, cover a broad spectrum of color indicators. The great advantageof this is that the chemistry does not change-that is, thestandard operation procedure generally does not have tobe adapted. In addition to the determination of chon-droitin sulfate (T-083) described below, bismuth nitrate(T-088), manganese sulfate (T-089), and zinc sulfate(T-090) can also be determined in accordance withPh. Eur. and USP. In accordance with Ph. Eur. and USP: photometric determina-tion of chondroitin sulfate using the Optrode (660 nm) and with1-hexadecylpyridinium chloride (also called cetylpyridinium chlo-ride, CPC) as titrant More information and more than 1800 other applica-tions can be downloaded free of charge at:www.metrohm.com/applications/ Water determination according to Karl Fischer The quality, effectiveness, and shelf life of pharmaceuti-cal products depends to a very great extent on theirwater content, which is why a great deal of importanceis attached to water determination in pharmaceuticalanalysis. Thanks to its specific and selective reaction withwater, Karl Fischer titration (KFT) is one of the most accu-rate and reproducible water determination methods,which is why numerous pharmacopoeias have prescribedit for years as a standard method for fast, automation-ready water determination. If the test substance is completely soluble in the KarlFischer reagent and if it does not enter into any side reac-tions with the solvent, the sample can be added directlyinto the titration cell. The water content can then bedetermined directly using volumetry or coulometry. Not all substances dissolve completely in methanol, butthat is an important prerequisite for correct determina-tion of a sample's water content. Some techniques forimproving the solubility of samples are listed below.These techniques can also be combined with one another. Solubility promoters Solubility promoters can be added, depending on thetest substance. For samples containing fat or oil, such asointments or creams, chloroform is added to the KFreagent to ensure that the sample dissolves completely. High-frequency homogenizers Tablets must be pulverized before titration. This caneither be done manually with a mortar or, more conve-niently and above all more reproducibly, using a high-frequency homogenizer directly in the closed titrationcell. The latter method prevents any changes to thewater content of the sample during preparation. In somecases, it can also eliminate the need for toxic solubilitypromoters. Higher temperatures in the titration cell Another option is titration of the water at a higher tem-perature (e.g., at 50 C). 901 Titrando with 900 Touch Control The 852 Titrando for volumetric and coulometric water determination 860 KF Thermoprep with 901 Titrando, 803 Ti Stand, and 900 Touch Control Karl Fischer oven method Many substances release their water only very slowly oronly at high temperatures. Some react with KF reagentsto form water or consume iodine, thus resulting in anerroneous determination of water content. Such samplesare unsuitable for direct Karl Fischer titration. Both theU.S. Pharmacopeia and the European Pharmacopoeiarequire determination of the drying loss in a drying ovenor desiccator for such substances. The drawback of thismethod, however, is that it determines not only thewater content, but also that of other volatile constituentscontained in the sample. In the KF oven method, the test substance is heated in atightly sealed vessel in an oven. The water driven offfrom the sample is transferred with a stream of dry car-rier gas into the titration cell, where it is determined as arule with coulometric Karl Fischer titration. The possibilityof side reactions and matrix effects can be excludedbecause the sample itself remains in the vessel and onlywater enters the titration cell. MATi 11 - fully automatic Karl Fischer titration including sample preparation Sample preparation 14 The complete range of automatic samplepreparation from a single source Normally, accurate pipetting and dilution of the sample isenough to determine the liquid forms of drugs (tinctures,infusions, drops, etc.). Metrohm offers you a wide rangeof products for automated, precise, and time-savingpreparation of liquid samples. Sample preparation becomes more demanding, however,when solid or semisolid samples are involved, i.e.,tablets, suppositories, and ointments. As a specialist inthe field of laboratory automation, we also offer you awide range of solutions for the preparation of solidsamples - customized to your needs upon request. Automation: time savings and more accurateresults Fully automated sample preparation and analysis of tablets:following the addition of a solvent, the Polytron high-frequencyhomogenizer pulverizes the tablets directly in the sample beaker.Every sample gets the same treatment. In addition to direct titration, chromatographic methodssuch as IC, HPLC, and GC are of primary use in pharma-ceuticals analysis. These techniques require that thesample is available as a filtered liquid before it is injectedon to the column. When carried out manually, as is oftenthe case, sample preparation steps such as pulverization and homogenization filtrationpipetting and dilution are tedious and time-consuming. Furthermore, manualsample preparation involves the risk of inaccurate results.Consistent sample preparation quality can hardly beguaranteed, particularly in cases of high sample through-put and when several people are involved. @ Wokulic USB Robotic Soliprep- automatic sample preparationtailored to your needs With the instruments of the Robotic Soliprep family, nei-ther deviation in results nor time-consuming manual rou-tines are an issue any longer. The solid substance is simplyweighed out and placed on the sample rack- everythingelse is completely automated. Depending on the versionselected, different steps can be combined -including thedirect connection to a chromatograph or the titration ofthe homogenized sample. Fully automated filtration: The 815 Robotic Filtration Soliprepfilters out residual solid matter from the homogenized sample.What remains is a clear filtrate that can be either injected directlyinto an analyzer or subjected to further dilution. RoboticTitrationSoliprep RoboticFiltration Soliprep RoboticFlexible Soliprep RoboticSoliprep for LC Homogenization + 十 + 十 Titration + Filtration + + + HPLC/GC vialfilling + Connection to an LC instrument s Ointments, lotions, and cosmetics Natural fats and oils have limited storage stabilitybecause they are slowly oxidized by atmospheric oxygen.The oxidation stability of fats and oils has been a defaultparameter in quality assurance for many years. To deter-mine oxidation stability with the Rancimat method, air ispassed through the test sample at a high temperature tocause artificial aging. During this process, the fatty acidsare oxidized by oxygen, forming volatile organic com-pounds and other products. These are driven off by theair flow and absorbed in water, where they are detectedwith conductivity measurement. The time it takes forthese decomposition products to form is known as theinduction time and characterizes the resistance of thesample to oxidative aging processes, i.e., its oxidationstability. The method is used to monitor natural fats and oils thatare used in the manufacture of aliphatic pharmaceutical products such as ointments, creams, and lotions. Inmany cases, however, it is also possible to investigate theoxidation stability of the finished formulations. For this towork properly, the proportion of fat in the sample mustbe significantly higher than the proportion of water. 892 Professional Rancimat and StabNet software The 892 Professional Rancimat enables simple and reli-able determination of oxidation stability in natural fatsand oils for up to eight samples. The instrument is con-trolled by a PC using StabNet software to plot measure-ment curves, evaluate them automatically, and calculatethe result. 192 Prelestisnal Waecinat Rancimat control with StabNet software:Up to eight samples can be analyzed simultaneouslyfor oxidation stability-and all in conformance with FDA and GLP requirements. Ion chromatography (IC) is the method of choice fordetermining active pharmaceutical ingredients, excipi-ents, traces of impurities, and metabolites in the form oforganic and inorganic ions or polar substances-not onlyin a broad range of pharmaceuticals and pharmaceuticalsolutions, but also in bodily fluids. Ion chromatographycan determine chemically similar substances in a veryshort time in just a single analysis. The concentrationrange of the analytes can extend from ng/L up to thepercentage range. Another advantage is the large selec-tion of separation columns and elution systems available.Interfering matrix effects can be avoided by using a suit-able inline sample preparation method or through thechoice of detection method: ·Conductivity detection with or without chemicalsuppression ·Amperometric detection ·Spectrophotometric detection- direct or withpost-column derivatization (UV/VIS) ·Coupled detection methods such as IC-MS andIC-ICP/MS 940 Professional IC Vario with 941 Eluent Production Moduleand 858 Professional Sample Processor: an intelligent ionchromatography system for parallel determination of anions andcations in pharmaceutical products The intelligent ion chromatographs with their automa-tion devices and peripheral equipment are controlled bythe user-friendly MaglC Net software. Complete docu-mentation on the status of the analytical instruments anduser activities enables complete traceability of the ana-lytical results. MaglC Net supports FDA Regulation21 CFR Part 11, and offers numerous tools for compli-ance with GLP guidelines. Metrohm ion chromatographs can also be operated withEmpowersoftware from Waters. In a certified environ-ment, uniform software minimizes training requirementsand reduces expenses associated with GLP compliance. Pharmaceutical solutions The term 《pharmaceutical solution》 denotes isotonicsolutions, hemodialysis solutions, or infusion solutions.They contain anions, cations,carbohydrates,and organicacids, the concentrations of which frequently differ fromone another by several orders of magnitude. In the con-text of production monitoring and final quality control,these ingredients need to be determined easily, quickly,and with a high degree of precision. Ion chromatographyis well up to this task with its intelligent analysis tech-nique and automatic inline sample preparation. Cation analysis of a solution of Ringer's lactate. Column:Metrosep C 4-100/4.0; Eluent: 1.7 mmol/L HNO,, 0.7 mmol/Ldipicolinic acid, 0.9 mL/min; sample volume: 10 pL; 1:20 (v/v)Inline Dilution Active pharmaceutical ingredients Active pharmaceutical ingredients in tablets such as gen-tamicin, neomycin, cefadroxil, or bethanechol chloridecan be determined byion chromatography in accordancewith the regulations of the U.S. Pharmacopeia andEuropean Pharmacopoeia. Requirements with regard toprecision, separation, and recovery of the analytes aredescribed in detail in the pharmacopoeias. IC determination of the antibiotic gentamicin with pulsedamperometric detection.Column: PolymerLaboratoriesRP-S; eluent: 60 g/L Na,SO, 1.75 g/L sodium octane sulfonate,1.34 g/L NaH,PO, 8 mL/L THF (pH=3, H,PO), 1.0 mL/min;column temperature: 55°C; sample volume: 20 pL; post-columnaddition: 300 mmol/LNaOH (0.4 mL/min) Impurities in pharmaceuticals In addition to active pharmaceutical ingredient analysis,ion chromatography can also be used to determineimpurities in pharmaceutical products. Even small con-centrations of an impurity can cause significant sideeffects. The azide used in the synthesis of the antihyper-tensive Irbesartan can be detected in trace amounts asan impurity in the product. The U.S. Pharmacopeia rec-ommends ion chromatographic azide determinationafter direct injection in accordance with USP<621>. Theazide determination is more selective, more sensitive,and most importantly- faster - when using Inline MatrixElimination, whereby the interfering pharmaceuticalmatrix is already separated from the target analyte duringthe sample preparation step. Irbesartan sample spiked with 5-80 pg/L azide; Column: Metrosep A Supp 10 -250/4.0; eluent: 5 mmol/L Na,CO,,5mol/L NaHCO,, 1.0 mL/min; column temperature: 60 C;sample volume: 1000 uL; Inline Matrix Elimination with 70:30(v/v) methanol/water Mean value [uS/cm] RSD [%] Recovery [%] 5 pg/Lspike 30 ug/L spike 0.4223 2.5754 1.96 101.71 103.38 0.14 Radiopharmaceuticals in nuclear medicine Radiopharmaceuticals are radioactive substances used indiagnostic procedures such as positron emission tomo-graphy (PET). They consist of an inert or biologicallyactive molecule bonded with a radioactive isotope, aso-called radionuclide. Radiopharmaceuticals participatein metabolic processes, which is why they are critical forgaining an understanding of biochemical and physiolo-gical processes in oncology, cardiology, and neurology. -[ Useful annihilation radiation PET is based on the fact that every time a nuclide decays,a positron is emitted that annihilates with its antiparticle,the electron. In doing so, the masses of each of theseelementary particles are converted directly into energy,thereby emitting two gamma rays in opposite directions.This so-called annihilation radiation can be detected withhigh-sensitivity detectors aligned in a ring surroundingthe patient. Positron emission tomography Computers use the PET data to calculate the origin of thegamma rays and thus the location where the radio-pharmaceutical decayed. A selection of available radio-)-pharmaceuticals and sophisticated detector systems annihilation image reconstruction Principle of positron emission tomography (PET): Disintegrationof the radionuclide releases two photons that can be sensitivelyregistered by coincident detection. By tracking the photons,powerful computers generate three-dimensional images of thephoton source. make it possible to create three-dimensional images of awide variety of tissues. This provides a unique picture ofphysiological, biochemical, and pharmacological pro-cesses taking place in the body, even at the molecularlevel. [F]Fluorodeoxyglucose (["F]FDG) [F]Fluorodeoxyglucose is a glucose analog in which theF nuclide is substituted for the hydroxyl group at the2'position. FDG, which makes possible the determina-tion of regional glucose consumption, is the most com-monly used radiopharmaceutical. Sophisticated quality control Quality control in radiopharmaceuticals is demanding,not least due to the strict time requirements, the safetyregulations, and the concentrations in the nanomolarrange. Metrohm's ion chromatographs fulfilltheserequirements and the regulations contained in manypharmacopoeias. A single multichannel radio ion chro-matograph is capable of performing quality control ondifferent production lines. In addition to the highestanalysis quality, ion chromatography provides users withsafety, low maintenance costs, and extreme robustness. lon chromatography from Metrohm has much more tooffer. The following table presents a selection ofadditional key determinations relevant to pharmaceutical quality control. If you do not see your application, pleasecontact your local Metrohm representative. Detection method: conductivity detection with suppression; direct conductivity detection; conductivity detection with andwithout suppression; amperometric detection; spectrophotometric detection Voltammetric trace analysis determines electrochemicallyactive substances. In many cases, these are traces ofheavy metals. Voltammetry is frequently employed tocomplement and validate spectroscopic methods. Itfeatures modest equipment requirements, relatively lowinvestment andoperating costs, simplee samplepreparation, short analysis times, and high accuracy and sensitivity. In addition, unlike spectroscopic methods,voltammetry can distinguish between different oxidationstates of metal ions as well as between free and boundmetal ions. This is referred to as speciation analysis.Voltammetric results provide important informationregarding the bioavailability and toxicity of heavy metals. Detection limits (1 ppt=1 ng/kg) Element Detection limit [ppt] Element Detection limit [ppt] Antimony Sb"/Sb 200 Molybdenum Mo 50 Nickel Ni 50 Arsenic As"/As’ 100 Platinum Pt 0.1 Bismuth Bi 500 Rhodium Rh 0.1 Lead Pb 50 Cadmium Cd 50 Mercury Hg 100 Chromium Cr/Cr 25 Selenium Se/SeM 300 Cobalt Co 50 Thallium TI 50 Iron Fe/Fe" 50 Uranium U 25 Copper Cu 50 Tungsten W 200 Zinc Zn 50 Voltammetry also enables organic compounds to bedetermined with a high degree of sensitivity. This makesit possible to analyze many active pharmaceuticalingredients in accordance with USP<801>. Voltammetry is particularly well-suited to laboratories inwhich only a few parameters need to be monitored incombination with a moderate sample throughput. It isfrequently used for special applications which cannot beperformed using other techniques or only with a greatdeal of effort. The 884 Professional VA is a flexible measuring instru-ment for accurate and sensitive polarographic voltam-metric analyses. The accompanying viva software enablesindividual optimization of methods, for example, theautomatic calculation of results according to special for-mulas prescribed by the USP. Fe(Il) in iron sucrose injection solution inaccordance with USP-NF Polarography makes it possible to determine, directly andselectively, the proportion of iron(Il) within the totalconcentration of iron in a preparation. No prior samplepreparation and chemical separation of the iron speciesis required. The separated signals of the iron(Il) andiron(III) complex are measured during determination. Theiron(II) content is derived from the ratio of the signals. Polarographic determination of iron(Il) content Thimerosal in accordance with USP-NF Mercury-containing thimerosal (or thiomersal) is used asa preservative for pharmaceuticals and cosmetics to pro-tect them from microbial contamination. Examples of theuse of thimerosal are: cleaning and storage solutions forcontact lenses; eye, nose, and ear drops; and tattoo inks.lnjectable medicinal products - for example, immuno-globulins and many vaccines (flu, hepatitis B, etc.)- canalso be preserved with thimerosal. Direct determination inthe finished preparation is possible in many cases. Heavy metal impurities For more than a century now, international pharma-copoeias have relied on sulfide precipitation for asemiquantitative determination of the heavy metalcontent in pharmaceutical products. As of 2018, this isno longer the case: USP requlations USP<232> andUSP<233> then require individual determinations ofselected heavy metals in place of sum parameters. TheUSP leaves it up to the user to select the analysis method,requiring only its validation. Stripping voltammetry isparticularly suitable here, and a simple sample digestionis sufficient for sample preparation. Other active pharmaceutical ingredients that canbe determined by polarography in accordance with USP-NF Electrochemically active pharmaceutical ingredients, suchas azathioprine, cefamandole, cysteine hydrochloride,diclofenamide, iodine, or procarbazine, can be determineddirectly with polarography in accordance with USP<801>. Electrochemistry for electroactive pharmaceuticals Electrochemistry in pharmaceuticals Many of the active pharmaceutical ingredients are elec-troactive, which makes them easy to determine usingelectrochemical methods. Electrochemistry is both highlysensitive and selective, and also has an outstandingdynamic range withvery rapididresponsetimes.Furthermore, there are numerous possibilities for record-ing signals. Potentiometric, amperometric, impedimetric,and electrogravimetric analyses are numbered amongthese. Real-time blood sugar monitoring With the development of amperometric glucose sensors- at the heart of every blood glucose meter - electro-chemistry has significantly improved the quality of life ofdiabetes patients. Basic research efforts are constantlybeing directed towards further improvements of thesetypes of sensors. The goal is to develop an implantablesensor for real-time monitoring. Research and development Electrochemical methods help to develop and character-ize new materials for use in biosensors and electroanalv-sis. In combination with proven electroanalytical meth-ods, these new, specially designed composite materials-mostly based on metal nanoparticles, carbon nano-tubes, or graphene - improve the sensitivity, specificity,and detection limits of measurements. High-end instruments from Metrohm Autolab Metrohm Autolab offers a range of high-end instrumentsfor electrochemical research. A broad array of modulesenables individual adaptation of the instruments to everydesired application. Electrochemical impedance spectros-copy measurements can be performed with the FRA32M,and the EQCM enables electrochemical quartz crystalmicrobalance measurements. For sensitive electrochemical impedance measurements in pharmacy: the Autolab PGSTAT204 with the FRA32M module www.metrohm-autolab.com 25 Sanofi avenie 《Why didl choose Metrohm? I havebeenworkingwithMetrohm for 25vears. When a new lab was to be builthere in Montpellier, we naturally choseMetrohm.》 Sandrine Caristan, Sanofi-aventis Sandrine Caristan and her teamprovide analytical support式Sanofi-aventis’R8Dcenter.MMontpellier, France. Determinationof the water content by Karl Fischertitrationisone of their mostfrequent analyses. A MetrcUSB Oven Sample Processor istheir solution of choice for higherthroughput and reliable results.And thanks to the client-serverversionofMetrohm'stiamosoftware, Sandrine doesn't have toleave her office to manage datafrom workplaces all over the site. Metrohm. People you can trust. testimonials.metrohm.com NIRS - interaction of light and physical matter Near-infrared spectroscopy (NIRS) is an analytical tech-nique based on the absorption of radiation by matter.Molecular vibrations are induced in the near-infraredregion of the magnetic spectrum (800-2500 nm) -i.e.,from the end of the visible to the mid-infrared (MIR)range. The main absorption bands of the functionalgroups of chemical substances are located in the MIRrange and are very strong. The absorption bands of theharmonics, however, and the combination of the funda-mental molecular vibrations are in the NIR spectralregion. They are significantly weaker and enable directmeasurement without sample preparation, while at thesame time offering deep insights into the chemical andphysical properties of the sample. The strongest overtoneabsorptions in the NIR range are displayed by com-pounds with OH, CH, NH, and SH bonds. Because theNIR spectrum represents the result of numerous overlap-ping absorption bands, it is normally evaluated withmultivariate chemometric methods. NIR spectra of lactose monohydrate and lactose anhydride foridentifying excipients Calibration model for the quantitative determination of theactive ingredient content of tablets; in this case, chlorpheniraminemaleate (CPM). NIR analysis establishes a connection betweenthe target value of a determination - in this case, CPM content- and the measured data in the spectrum. Many parameters in a single analysis NIRS offers numerous advantages over many wet-chem-ical analytical methods. A diverse range of parameterscan be determined simultaneously with just one analysis.NIRS is economical and fast, enabling qualitative andquantitative analyses that are noninvasive and nonde-structive. NIRS is an indispensable analysis technique that can beused along the entire production chain - from incomingmaterials to processing to the quality control of finishedproducts. NIRS meets the requirements of numerousinternational pharmacopoeias, e.g.,USP, Ph. Eur., and JP. How NIRS - the fast solution for every sample matrixNear-infrared spectroscopy requires no sample prepara-tion and can handle any sample matrix, no matter if it is: · powders or granulates · tablets or capsules · creams or gels solutions or suspensions ·polymer films ·freeze-dried samples. Where What Incoming goods inspection of raw materials starting from step one Incomingmaterials ·Identity tests directly in the warehouse for active pharmaceutical ingredients, excipients,and packaging · Quality control (purity, chemical and physical properties) Real-time monitoring, optimizing the drying process Drying · Inline determination of water and solvents in powders and granulates (page 33)·Determination of the endpoint of drying processes ·Determination of residual water content in freeze-dried products Monitoring blending processes Blending andgranulation ·Determination of blend homogeneity · Setting required granulation time by tracking blend quality Faster analytical results and minimization of rejected products Tablet pressingand capsule filling · Content uniformity test in solid dosage forms (tablets and capsules) ·Determination of tablet characteristics (hardness, stability,etc.) · Identification of tablets and products before they are packaged Final productcontrol andpackaging Less expended effort compared to reference methods (e.g., HPLC) ·Content determination in finished products (creams, gels, solutions, tablets, capsules)· Final product inspection NIRS - screening through packaged materials NIRS can even perform determinations on contentssealed in transparent packaging such as glass and films.This is particularly appealing for incoming goods inspec-tions and packaged end products. Handling could not beeasier; in fact, it is so easy that NIRS can be used directlyin pharmacies and customs offices. 28 Nondestructive analysis technique NIRS has long been one of the most important and ver-satile analytical techniques in the pharmaceutical indus-try - and not just because everybody in the pharmaindustry is talking about PAT and QbD. The decisivebenefit of NIRS is the possibility of obtaining reliableanalysis results in just seconds without any sample prepa-ration or reagents whatsoever. PAT and QbD- with NIRS in search of the best ofall methods Drug manufacturing is undergoing a transformation.TheFDA's stated goal is to cut development time for newdrugs while at the same time significantly improving qual-ity. This requirement can only be fulfilled with analyticaltechniques which monitor the entire process - fromincoming raw materials to the final inspection. To achievethat, perfect PAT sensors are needed that enable "live"tracking of the manufacturing process. NIRS is the tech-nique that makes this possible. An inline sensor monitorsproduct quality in real time (see also pages 32 and 33). This prevents charges related to rejected products andreduces overall costs. In accordance with international pharmacopoeiasAs a secondary test method, NIRS is recommended in allof the key pharmacopoeias - from the European(Ph. Eur. 2.2.40) to the American (USP<1119>) to theJapanese pharmacopoeia. All of Metrohm's NIRSystemsinstruments meet the standards for wavelength preci-sion, reproducibility, and photometric noise. Numerousreference standards and user-friendly software make iteasy to check the instrument requirements specified inthe pharmacopoeias. The pharmaceutical version of theVision software is fully validated and compliant with 21CFR Part 11. Metrohm NIRSystems also offers complete IQ/OQ docu-mentation and instrument performance certification(IPC). Documented parameters guarantee that the instru-ment performs properly. Atline, online, and inline analysis systems fromMetrohm Process Analytics Atline, online, and inline analysis systems from MetrohmProcess Analytics are the preferred solution for processmonitoring in a wide range of industries. Reliable analysisresults are determined directly in-process with the latestmethods of ion analysis and spectroscopy: Measurementof pH value, conductivity, and redox potential, as well asTOC, titration, Karl Fischer titration, photometry, ion-selective electrode measurement (dynamic standardaddition), ion chromatography, voltammetry, and near-infrared spectroscopy. Metrohm Applikon, with the brand name MetrohmProcess Analytics, is an inline, online, and atline analysis specialist with more than 40 years' experience in thefield. We offer a broad program of process analyzers andsample preparation systems for a large array of applicationsin a wide number of industries. Metrohm Applikon- globally present Metrohm Applikon is part of the Metrohm Group sup-porting you globally with offices in 45 countries. Ourspecialists offer you advice during the planning anddevelopment of your own custom-designed analysis sys-tem, commission the system, and provide professionalmaintenance and service during routine operations. More efficient production with PAT and QbD Drugs are manufactured in accordance with very strictdirectives and must meet high standards with respect toquality, effectiveness, and safety. Every process step isthoroughly monitored during the manufacture of variousactive ingredients and excipients. Added to that areexhaustive approval analyses at the end of the manufac-turing process: pharmaceutical producers often spendmore time on final checks than on the production itself.Stringent regulatory requirements also make it more dif-ficult to optimize pharmaceutical manufacturing pro-cesses. With its Process Analytical Technology (PAT) initia-tive and the principle of Quality-by-Design (QbD), theFDA is striving to increase the efficiency of drug produc-tion. The approach involves a departure from final in-spections toward real-time process analysis and checks. Rapid process monitoring Metrohm Process Analytics offers a robust analysis sys-tem that is easy to operate and can be set up directly onthe production floor. The sample is brought to theProcessLab and the analysis started at the push of a sin-gle button. ProcessLab is based on the proven Titrandosystem, which in combination with tiamo software meets the requirements of the FDA Regulation 21 CFRPart 11. ProcessLab features a modular design and isconfigured to meet specific analytical requirements. Itcan be optimally integrated into process communicationsthrough inputs and outputs (typically 4-20 mA). Sampleidentification is further simplified through the use of abarcode reader. Just minutes after the sample is collect-ed, the relevant process information is available to a LIMSor the master display. Atline analyzer ADI 2045PL ProcessLab The ADI 2045PL ProcessLab atline analyzer is ideallysuited to rapid and independent process monitoring inthe production environment. A ProcessLab analysis sys-tem consists of a TFT touch screen operating unit and ananalysis module that is tailored to the specific applica-tion. Thanks to its splashproof housing (housing protec-tion class IP66/NEMA 4), ProcessLab is ideally suited toharsh production environments. The pharmaceuticalindustry is held to high standards of hygiene, which iswhy most equipment is made with a stainless steel hous-ing. ADI 2045PL ProcessLab: each system is configured with the relevant modules according to user preferences. Analysis of acidic and basic components Numerous pharmaceutical intermediates and end pro-ducts contain acidic and basic components. These aredetermined with a suitable acid-base titration method.Depending on the sample matrix, either aqueous or non-aqueous titrations are carried out. The latter are donewith a titrant and solvent suitable for the particular appli-cation- for example, with perchloric acid in glacial aceticacid or with tetrabutylammonium hydroxide (TBAH) in2-propanol or acetone. Determination of redox-sensitive components Oxidizable and reducible components and active ingredi-ents are determined with redox titrations. Methods fre-quently used include iodometry, cerimetry, bromato-metry, and/or permanganometry. The modular structureof ProcessLab enables easy adaptation to the specificrequirements of the application. Because of its proximityto the process, the relevant analytical values are availablewithin just a few minutes. Online and inline process analysis Customized online process control Production processes in the pharmaceuticals industry mustbe continuously monitored. Online and inline analyzersfrom Metrohm Process Analytics optimally fulfill thisrequirement. Engineered for continuous operation, theseinstruments enable fully automatic checks of productionprocesses - around the clock, seven days a week.Moreover, it does not make a difference whether a singleparameter is to be determined in a single sample streamor several different parameters are to be determinedsimultaneously in complex, multiple sample streams -Metrohm Process Analytics provides you with a suitablesystem for all applications. Proven wet chemistry methods Metrohm Process Analytics online analyzers are based onwet chemistry processes such as titration, colorimetry,and measurements with ion-selective electrodes; near-infrared spectroscopy is used for inline monitoring.Sampling and sample preparation are at least as impor-r-tant as the analysis itself. Metrohm Process Analytics hasgreat expertise in this field and configures the samplingsystem to fit your application precisely, including featuressuch as filtration, the removal of samples from pressur-ized containers, and degassing. TOC analysis in real time Determination of total organic carbon (TOC) content isamong the routine procedures in the pharmaceuticalindustry. TOC enables quality monitoring of water in realtime. It also shows the progress of cleaning proceduresin the production equipment. Straightforward network integration All Metrohm Process Analytics online and inline analyzersare equipped with digital and analog data outputs.lResults can be transmitted via analog 4-20 mA signalsand alarms can be triggered by digital outputs. Digitainputs can be employed for remote start/stop com-mands. Robust design in stainless steel Metrohm Process Analytics analyzers are constructed forthe demands of the harsh production environment. Thehousings meet NEMA 4 and protection class IP66 speci-fications. In environments that demand the highest stan-dards of hygiene and durability, Metrohm ProcessAnalytics offers its ADI 2045TI and ADI 201Y ProcessAnalyzers equipped with stainless steel housings. Inline process analysis with NIRS The goal of the FDA's PAT/QbD approach is process opti-mization through improved process controls. Instead oftime-consuming final inspections, PAT calls for monitor-ing product quality in real time. Near-infrared spectros-copy offers meaningful help toward achieving this objec-tive: NIR always has the process in view, it is not destruc-tive, requires no sample preparation, and delivers preciseresults in just seconds. The software uses multivariatedata analysis to extract the data required for the analysisat hand from the comprehensive NIR data. Physicalparameters, such as density and particle size distribution,in addition to water or solvent content, can also be con-veniently determined. Eye on the process: granulation and drying A key manufacturing process in the pharmaceuticalindustry is the granulation and drying process for pow-ders that precedes tablet manufacturing and makes itpossible to press powders into tablets in the first place.NIRS is the method of choice for determining the reac-tion endpoint when pressability is at the optimal point.Probes in the drier or granulator make it possible to trackthe process in real time. That reduces the process dura-tion and thus increases the drying and granulation capac-ity of the system. At the same time, it minimizes thedeviation from the required setpoint values. Calibration model for quantitative determination of water con-tent in powders. Karl Fischer titration is the reference method forthe determination of the water content. Reduction of water content in a pharmaceutical powder over time. Rapid and nondestructiveNIR analysis makes it possible to determine the optimal moment for further processing in realtime. The sensor is installed directly in the granulator. Metrohm Quality Service - Service you can rely on Reliable results - for the lifetime of the analyzer Particularly in the pharmaceutical industry, measurementerrors can have serious consequences and must there-fore be avoided at all cost. The name Metrohm standsthroughout the world for high-quality laboratory analyz-ers for ion analysis. They are designed to deliver excep-tionally precise results. Leading international pharmaceu-tical companies also value Metrohm for our comprehen-sive service, which ensures that the users can rely ontheir results for the entire lifetime of their instruments. With Metrohm Quality Service you are on the safe sidefrom day one. From installation and start-up, to regularmaintenance and fast repair- if problems arise - weguarantee that you can rely on your instrument and gainmaximum uptime from your investment. You can trust Metrohm Compliance Service when it istime for the professional qualification of your analyzers.The wide range of different laboratory instruments andanalyzers makes it very complicated for pharmaceuticalcompanies to keep in step with legal requirements. As anexperienced and reliable partner, Metrohm provides itscustomers with technical advice and expertise, withrespect not only to instruments and applications, butalso to the latest regulatory requirements. Analyzers used in laboratories must satisfy the latestrequirements of FDA requlations, GLP/GMP standards,and the GAMP directives. This is achieved through instru-ment qualification and system validation. Metrohm offers documents for analytical instrument quali- fication (AIQ) as well as services that Metrohm Quality Service Metrohm’s global Quality Service, and regularly sched-uled preventive maintenance in particular, extends yourinstrument's lifetime and ensures trouble-free operation.Maintenance work is carried out by qualified and certi-fied service engineers. You have the option of selectingdifferent types of service contracts depending on your particular need. With a Total Care Contract, for example,you can rely on the optimum performance of yourMetrohm instruments at all times, incurring no additionalcosts whatsoever and benefit from complete and com-pliant documentation. Ordering information 36 pH value and conductivity measurement pH value measurement 2.867.0110 867 pH Module with Touch Control 2.867.0210 867 pH Module with tiamo light Conductivity measurement 2.856.0120 856 Conductivity Module with Touch Control and stainless steel measuring cell 2.856.0220 856 Conductivity Module with tiamo light and stainless steel measuring cell 6.0916.040 Conductivity measuring cell made of stainless steel, c = 0.1 cm-l, with Pt1000 Titration 2.907.1020 Pharm Titrando 2.902.0010 902 Stat Titrando 6.6056.2X2 tiamo 2.X full Water determination according to Karl Fischer Coulometric KF Titration 2.756.0010 756 KF Coulometer with generator electrode with diaphragm 2.756.0110* 756 KF Coulometer with generator electrode without diaphragm2.831.0010 831 KF Coulometer with generator electrode with diaphragm2.831.0110* 831 KF Coulometer with generator electrode without diaphragm2.851.0010 851 Titrando with generator electrode with diaphragm2.851.0110* 851 Titrando with generator electrode without diaphragm 2.852.0050 852 Titrando with generator electrode with diaphragm and volumetric titration cell 2.852.0150* 852 Titrando with generator electrode without diaphragm and volumetric titration cell* The magnetic stirrer has to be ordered separately. Volumetric KF Titration 2.890.0110 890 Titrando with Touch Control 2.890.0210 890 Titrando with tiamo light 2.901.0010 901 Titrando including titration cell MATi 11 Automated volumetric Karl Fischer titration including sample preparation KF oven 2.860.0010 860 KF Thermoprep 2.874.0010 874 Oven Sample Processor Automation MATi 03 Automatic titration system for nonaqueous titrations in the pharmaceutical industry in series ofup to 59 samples 2.815.1110 815 Robotic Titration Soliprep 2.815.2110 815 Robotic Flexible Soliprep 2.815.3110 815 Robotic Filtration Soliprep 2.815.4110 815 Robotic Soliprep for LC 2.892.0010 892 Professional Rancimat including accessories, without StabNet software 6.6068.102 StabNet 1.0 Full 6.6068.103 StabNet 1.0 Multi 37 -1P.M. Ion chromatography 2.940.2500 940 Professional IC Vario TWO/SeS/PP for anion and cation determination 2.940.1520 940 Professional IC Vario ONE/SeS/PP/Prep2 2.850.9010 IC Conductivity Detector 2.850.9110 IC Amperometric Detector 2.858.0020 858 Professional Sample Processor for the automation of determinations 6.5337.010 IC Equipment Wall-Jet cell for carbohydrate determination 6.1090.420 Metrosep Carb 2-150/4.0 for carbohydrate determination 6.1050.410 Metrosep C 4- 100/4.0 for cation determination 6.1020.030 Metrosep A Supp 10-250/4.0 for anion determination Voltammetry 2.884.0110 884 Professional VA manual for Multi-Mode Electrode (MME) 2.884.1110 884 Professional VA semiautomated for MME consisting of 884 Professional VA, measuring head for MME and two 800 Dosinos. MVA-22 Fully automated Professional VA system consisting of 884 Professional VA, measuring head for MME,919 IC Autosampler plus for VA and two 800 Dosinos for automatic addition of auxiliary solutions. Allows the automatic processing of up to 28 samples. This system is the optimum solution forautomatic analysis of small sample series. Required Accessories 6.5339.030 VA-Elektrodenkit with Multi-Mode Electrode 6.6065.202 viva 2.0 Full Process analysis We offer online and atline Analyzers that meet any requirement in the process industry, from single-parameter tothe most advanced multiparameter analyzers. Every analyzer is custom-tailored to the specific task at hand. Wet chemistry ADI 2045PL ProcessLab system for atline determination of various parameters using titration, colorimetry,and ISE ADI 201Y Series Single method Process Analyzers, available with titration, colorimetry, or ISE ADI 204Y Series Multifunctional Process Analyzers, available with titration, ISE, colorimetry, and voltammetry2035 Series Analyzer family designed in 3 configurations: potentiometric, photometric, and thermometrictitration 7010 TOC Analyzer for determination of the total organic carbon content (TOC) in liquid samples Furthermore, we offer the Plug and Analyze Series -ICON and Alert for single method, single component wateranalysis. Near-infrared spectroscopy 2.928.0210 NIRS XDS Process Analyzer -SingleFiber SinglePoint 2.928.0220 NIRS XDS Process Analyzer -SingleFiber 4 Channels 2.928.0230 NIRS XDS Process Analyzer-SingleFiber 9 Channels 2.928.0110 NIRS XDS Process Analyzer - Microbundle SinglePoint 2.928.0120 NIRS XDS Process Analyzer - Microbundle 4 Channels 2.928.0130 NIRS XDS Process Analyzer - Microbundle 9 Channels 2.928.0310 NIRS XDS Process Analyzer -DirectLight/NonContact 2.928.1110 NIRS Analyzer PRO-ContactReflection 2.928.1120 NIRS Analyzer PRO - FiberSystem 2.928.1130 NIRS Analyzer PRO - DirectLight/NonContact Please contact your Metrohm supplier for further information. Please also consult www.metrohm.com pharma.metrohm.com coo 瑞士万通在制药行业的应用EN行业:制药绑定仪器:852、905、940关键词:电位滴定、卡休水分仪、氧化稳定性测定、离子色谱、近红外光谱、拉曼光谱、伏安极谱仪详细应用请下载资料了解。
关闭-
1/40

-
2/40

还剩38页未读,是否继续阅读?
继续免费阅读全文产品配置单
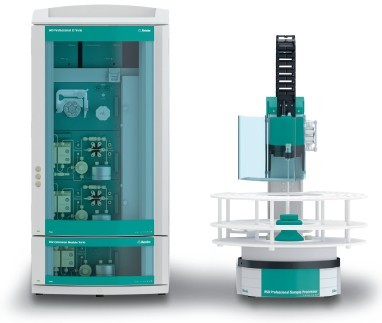
瑞士万通中国有限公司为您提供《药品中各种常见指标等检测方案(卡氏水分测定)》,该方案主要用于化药制剂中理化性质检测,参考标准《暂无》,《药品中各种常见指标等检测方案(卡氏水分测定)》用到的仪器有瑞士万通852 精湛一代库仑法/容量法 卡氏水份滴定仪、瑞士万通940 系列谱峰思维TM离子色谱系统、电位滴定仪-905 爱 智能系列全自动电位滴定仪 瑞士万通。
我要纠错
推荐专场
卡氏水分测定仪/卡氏水份测定仪
更多相关方案











 咨询
咨询




