方案详情文
智能文字提取功能测试中
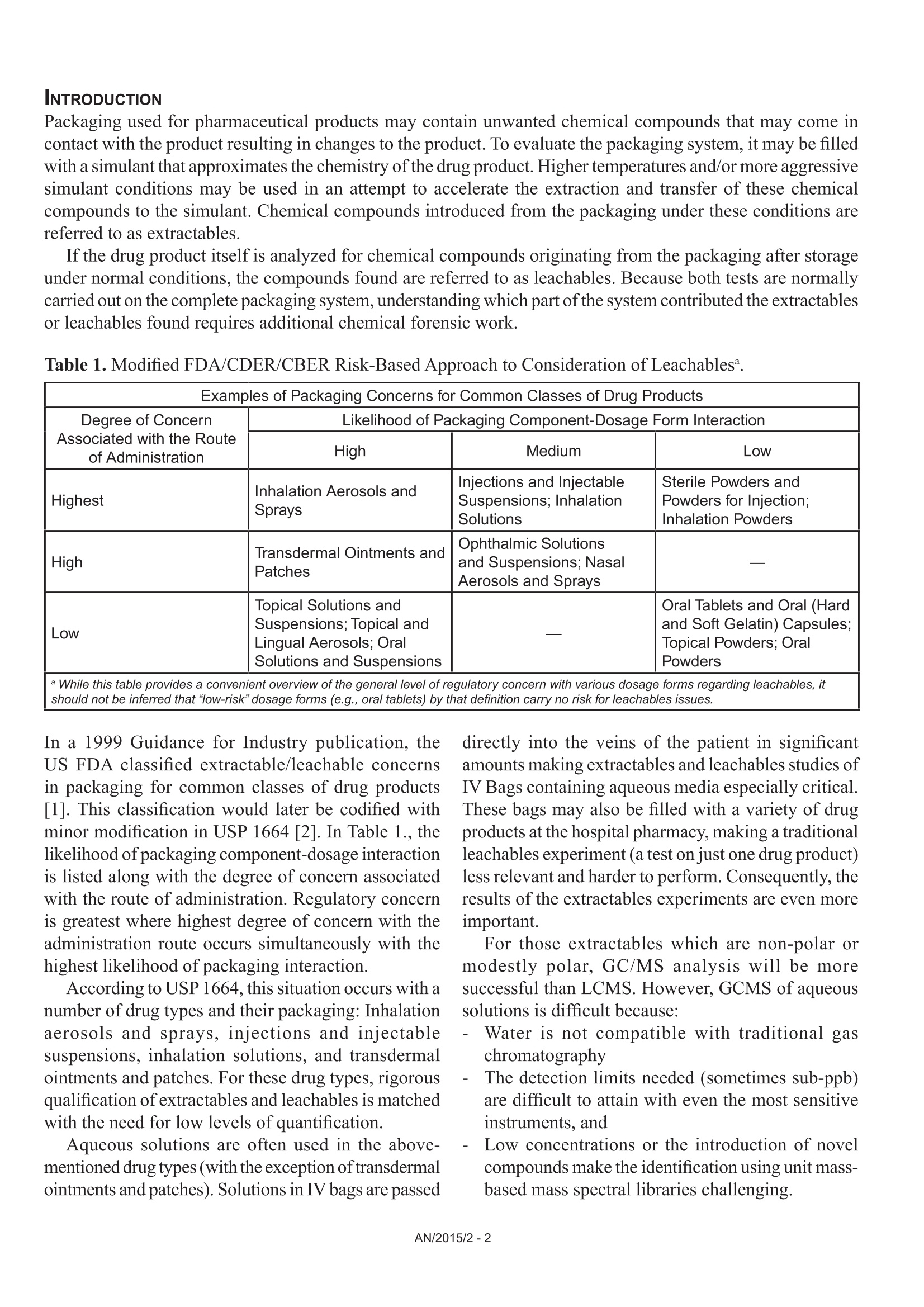
GLC)BBAAL A N AAL YTIC A S O)L U ODN S Figure 2 shows the resulting chromatogram following thermal extraction of 16.3 mg ofIV bag material, split 1:30. Extractables and Leachables Analysisof IV Bag Systems using Direct ThermalExtraction of the Materials and StirBar Sorptive Extraction of AqueousSolutions coupled with ThermalDesorption Gas-Chromatography withUnit Mass and High Resolution MassSpectrometric Detection Andreas Hoffmann, Thomas AlbinusGerstel GmbH & Co. KG, Eberhard-Gerstel-Platz 1,D-45473 Miilheim an der Ruhr, Germany Elizabeth AlmasiAgilent Technologies, 5301 Stevens Creek Boulevard,Santa Clara, CA 95051, USA Kurt Thaxton Gerstel,Inc., 701 Digital Dr. Suite J,Linthicum, MD 21090, USA KEYWORDS Pharmaceutical, Packaging, IV bags, intravenous deliverysystems, Extractables, Leachables, Single Quad MassSpectrometry, Time-of-Flight Mass Spectrometry, Stir BarSorptive Extraction (SBSE), Thermal Desorption, ThermalExtraction, Gas Chromatography ABSTRACT IV bag components were analyzed for extractables usingdirect thermal desorption/thermal extraction combined witha unit mass resolution GC/MSD system. The results werecompared to those obtained for leachables by stir bar sorptiveextraction of an aqueous simulant stored in the exact sametype ofIV bag, again combined with GC/MS determinationof the leached compounds. In addition, the high resolutionGC/Time of Flight (TOF) mass spectrometer was used toverify or disprove some of the MSD findings. INTRODUCTION Packaging used for pharmaceutical products may contain unwanted chemical compounds that may come incontact with the product resulting in changes to the product. To evaluate the packaging system, it may be filledwith a simulant that approximates the chemistry of the drug product. Higher temperatures and/or more aggressivesimulant conditions may be used in an attempt to accelerate the extraction and transfer of these chemicalcompounds to the simulant. Chemical compounds introduced from the packaging under these conditions arereferred to as extractables. If the drug product itself is analyzed for chemical compounds originating from the packaging after storageunder normal conditions, the compounds found are referred to as leachables. Because both tests are normallycarried out on the complete packaging system, understanding which part of the system contributed the extractablesor leachables found requires additional chemical forensic work. Table 1. Modified FDA/CDER/CBER Risk-Based Approach to Consideration of Leachables Examples of Packaging Concerns for Common Classes of Drug Products Degree of ConcernAssociated with the Routeof Administration Likelihood of Packaging Component-Dosage Form Interaction High Medium LOW Highest Inhalation Aerosols andSprays Injections and InjectableSuspensions; InhalationSolutions Sterile Powders andPowders for Injection;Inhalation Powders High Transdermal Ointments andPatches Ophthalmic Solutions and Suspensions; NasalAerosols and Sprays 二 LOW Topical Solutions and Suspensions; Topical andLingual Aerosols; Oral Solutions and Suspensions Oral Tablets and Oral (Hardand Soft Gelatin) Capsules;Topical Powders; Oral Powders aWhile this table provides a convenient overview of the general level of regulatory concern with various dosage forms regarding leachables, itshould not be inferred that“/ow-risk" dosage forms (e.g., oral tablets) by that definition carry no risk for leachables issues. In a 1999 Guidance for Industry publication, theUS FDA classified extractable/leachable concernsin packaging for common classes of drug products[1]. This classification would later be codified withminor modification in USP 1664 [2]. In Table 1., thelikelihood of packaging component-dosage interactionis listed along with the degree of concern associatedwith the route of administration. Regulatory concernis greatest where highest degree of concern with theadministration route occurs simultaneously with thehighest likelihood of packaging interaction. According to USP 1664, this situation occurs with anumber of drug types and their packaging: Inhalationaerosols and sprays, injections and injectablesuspensions, inhalation solutions, and transdermalointments and patches. For these drug types, rigorousqualification ofextractables and leachables is matchedwith the need for low levels of quantification. Aqueous solutions are often used in the above-mentioned drug types (with the exception oftransdermalointments and patches). Solutions in IV bags are passed directly into the veins of the patient in significantamounts making extractables and leachables studies ofIV Bags containing aqueous media especially critical.These bags may also be filled with a variety of drugproducts at the hospital pharmacy, making a traditionalleachables experiment (a test on just one drug product)less relevant and harder to perform. Consequently, theresults of the extractables experiments are even moreimportant. For those extractables which are non-polar ormodestly polar, GC/MS analysis will be moresuccessful than LCMS. However, GCMS of aqueoussolutions is difficult because: Water is not compatible with traditional gaschromatography The detection limits needed (sometimes sub-ppb)are difficult to attain with even the most sensitiveinstruments, and Low concentrations or the introduction of novelcompounds make the identification using unit mass-based mass spectral libraries challenging. The first two issues can be addressed using Stir BarSorptive Extraction (SBSE), a technique commonlyused in pharmaceutical and other applicationswhere concentration of analytes and extraction ofanalytes from the aqueous matrix is necessary [3, 4].Analytes are absorbed and concentrated on the StirBar (“Twister") and are subsequently desorbed usinga thermal desorption system, re-concentrated in aProgrammed Temperature Vaporizer (PTV) type GCinlet, and are then injected onto the GC column. The concentration factor achieved by usingSBSE-TD-GCMS often resolves some of the thirdissue, but sometimes does not. Where the use of unitmass spectrometry is insufficient, highly sensitivequadrupole time-of-flight mass spectrometers (QTOF-MS) can be used instead. In this work we were confronted with the task oftrying to determine the extractables from an IV bagsystem while at the same time understanding whichpart of the system they were introduced from (theleachables would be specific to a drug product to betested at a future time). EXPERIMENTAL To solve this issue, the approach we took was: 1. Direct thermal desorption (extraction) of bagcomponents at elevated temperatures 2. SBSE-GC/MS of an aqueous simulant stored in thebag at slightly elevated temperatures 3. Use of SBSE-GC-QTOF-MS where needed toresolve any qualification issues not solvable in steptWO. Step 1 is extremely important: direct desorption athigh(200°℃) temperatures ofthe individual bag componentsprovides a ‘menu’of extractables candidates forthe leachables analysis/simulation in step 2. Moreimportantly, direct thermal desorption of individual IVsystem components makes it easier to assign a detectedcompound to the bag component from which it couldbe leached. Materials and instrumentation. Empty and sterile, 250mL capacity IV bags were provided by a customer. Thebags were made from polypropylene, but, as indicatedon the outer packing, "some product componentscontain DEHP-plasticized PVC". Analysis of IV bag components were performedon a 7890B GC coupled with a 5977A MSD (AgilentTechnologies), equipped with a PTV Inlet (CIS 4),Thermal Desorption Unit (TDU), and MultiPurposeSampler (MPS) (all from GERSTEL). For the SBSE experiments Twister stir bars (GERSTEL)coated with 24 pL PDMS were applied. For desorptionofthe Twister the above-mentioned instrumental setupwas used. The confirmatory analyses were done on a 7890BGC coupled with a 7200 QTOF (Agilent Technologies),also equipped with a PTV Inlet (CIS 4), ThermalDesorption Unit (TDU), and MPS robotic sampler(GERSTEL). Direct thermal desorption (extraction) of IV bagcomponents Sample preparation. Small sample pieces (between 3and 15 mg) were taken from the IV bag at the positionsdisplayed in figure 1. These materials were then placedin empty, pre-conditioned TDU tubes for subsequentthermal extraction. Figure 1. IV bag sampling spots. GC/MS analysis. IV bag component samples placedin empty, pre-conditioned TDU tubes, were heated fordirect thermal extraction at temperatures of 80, 140,and 200°C. During this process volatilized analytes arepurged with the carrier gas into the pre-cooled CIS,concentrating the extracted compounds in the inlet forsubsequent GC-MS analysis. Thermal Desorption TDU (GERSTEL) Stir Bar Sorptive Extraction (SBSE) of an aqueoussimulant Sample preparation. A 250 mL volume of deionizedwater was filled into an empty IV bag and storedfor 48 hours at a slightly elevated temperature of40°℃ to allow compounds to leach into the aqueoussolution. After this period, a 10 mL aliquot of thewater was transferred to an empty vial. A Twister stirbar was added and the vial was capped. The samplewas extracted for 60 minutes through stirring with aTwister at room temperature. The stir bar was removed,rinsed with bottled water, dabbed dry and placed intoa conditioned thermal desorption tube for analysis. GC/MS analysis conditions. The Twister was desorbedat 240℃. Only those parameters that differ from thethermal extraction experiments are displayed. RESULTS AND DISCUSSION Please note: the evaluation of the data below is fordemonstration purposes only, and is not in any waya thorough evaluation of all the analytes observed.A complete account would require more time andresources than was available for this project. Direct thermal desorption (extraction) of IV bagcomponents. Thermal extraction of solid materials at hightemperatures allows analysis of volatile and semi-volatile species independent of their polarity. The easeof use and the efficiency of this technique enable fastand substantial extractables studies as the most relevantcompounds, those with relatively high mobility, arecovered. For extractables analysis this is a majoradvantage over solvent extraction, in which analyteextraction rates depend more on the polarity of thesolvent than on their mobility [5]. This procedureminimizes sample preparation and eliminates samplecontamination from solvents. 3 Figure 2. Thermal extraction of 13.6 mg IV bag material (polypropylene) at 80°℃, split 1:30. Cyclohexanone is often used as solvent in PVC production. A recent study of plastic tubing used in medicalprocedures that circulate blood outside the body suggests a link between this compound and decreased heartfunction, swelling, loss of taste and short term memory loss [6]. Almost all 2-Ethylhexanol produced globallyis converted into the diester bis(2-ethylhexyl) phthalate (DEHP), a plasticizer. Neither compound is typicallyrelated to the production of polypropylene, the material used to make this IV bag. The experiment was repeated, this time using an extraction temperature of200°C. Figure 3 shows an overlayof the extraction at 80°℃ (black trace) and 200°C (red trace). Figure 3.Overlay of 80C extraction (black trace) and 200°℃ extraction (red trace). The resulting chromatographic trace shows an increase, not only in recovery of semi-volatile compounds, whichwas to be expected, but also in recovery of volatile compounds (compounds eluting before cyclohexanone,0-8mins). Apparently these compounds (mainly residual solvents) are only released from the material by applyinghigh extraction temperatures. Figure 4 shows this section in detail. -w 2.00 3.00 6.00 6.50 7.00 .50 Figure 4. Thermal extraction of 15.1 mg IV bag material (polypropylene) at 200°℃, split 1:30, early elutingcompounds. The next IV bag component analyzed was made of the same material, but the sample was taken from a part ofthe bag onto which text had been printed (see figure 1). When we compared this result with the one from samplewithout printed information by overlaying the chromatograms, three additional peaks were found (figure 5). No. Compound Methyl methacrylate2 o-Toluenesulfonamide 3 p-Toluenesulfonamide 2 3... 5.00 10.00 15.00 20.00 25.00 30.00 35.00 40.00 45.00 Figure 5. Overlaid chromatograms of thermal extraction of 15.1 mg IV bag material (black trace) and 15.4 mgof IV bag material with imprint (red trace) at 200°C, split 1:30. The additional peaks were identified as methyl methacrylate, a chemical that is used among other things forthe production of paints and lacquers,as well as o- and p-toluenesulfonamide. The latter ones are commonlyused as plasticizers in coatings, paints, and printing inks. In addition, they promote adhesion and have excellentthermal stability. These compounds have the potential to cause damage to DNA (as genotoxic impurities). Theyare of highlighted concern and ultra-low level detection is highly desirable. Due to the high content of DEHP in the IV tubing material (most probably PVC) the thermal extraction experimentwas carried out at only 140°C. The resulting chromatogram is shown in figure 6. Figure 6. Thermal extraction of 3.7 mg IV tubing (PVC) at 140℃, split 1:30. The presence of cyclohexanone and 2-ethylhexanol in the chromatogram seems very plausible: The IV tubingis made of PVC containing high amounts of DEHP plasticizer. Cyclohexanone is a solvent that is often usedduring PVC production and 2-ethyl hexanol is an intermediate product in DEHP production. The analysis ofthe plastic valve, also performed at 140℃, showed a similar result (figure 7). This suggests that the valve andIV tubing are made of similar materials. Figure 7. Thermal extraction of 3.5 mg IV plastic valve (PVC) at 140°℃, split 1:30. The major difference in the plastic valve is a higher BHT concentration plus the presence of diethyl phthalate(DEP), a commonly used plasticizer. Comparing these results with those of the IV bag material we arrivedat the conclusion that compounds from the tubing and/or the plastic valve must have migrated into the bagmaterial and contaminated it. The following chromatogram demonstrates how high the DEHP content of the PVC components is comparedto the other volatiles of this sample. 2.3 mg of the plastic valve material were thermally extracted at 200°℃ andintroduced to the GC column with a split ratio of 1:30 (figure 8). Figure 8. Thermal extraction of 2.3 mg IV plastic valve (PVC) at 200℃, split 1:30. Although the sample size was reduced significantly to only 2.3 mg and the extracted volatiles were introducedto the GC column with a split ratio of 1: 30, the column is overloaded with DEHP and the peak is stretched outover 10 minutes. This shows that an absolute precondition for successful direct extraction experiments is aninert sample flow path. This is the only way to ensure that sample to sample carry over will not be experiencedand will not compromise the analytical results even for compounds present at very high concentration levels . Figure 9 shows an overlay of the overloaded chromatogram with a blank run with an empty sample tubeperformed immediately after the analysis using exactly the same instrument conditions. As can be seen, thereis virtually no sample to sample carry-over; therefore, even samples with high concentrations of SVOC’s canbe analyzed without impacting the accuracy of subsequent analyses. 55.00 60.00 Figure 9.Overlay chromatograms resulting from thermal extraction of 2.3 mg IV plastic valve material (PVC)at 200°C, split 1:30 (black trace) and a subsequent blank run (red trace) performed immediately after using thesame conditions. The blank run demonstrates that there is virtually no sample-to-sample carryover. Stir Bar Sorptive Extraction (SBSE) of an aqueous simulant. Stir bar sorptive extraction (SBSE) is a solvent-less sample extraction technique for enrichment of solutes fromaqueous samples, it was first introduced by Baltussen et al. in 1999 [7]. SBSE minimizes sample preparationand avoids sample contamination from organic solvents that are usually required to extract analytes from waterbased samples. In addition, there is no need for further concentration procedures, such as large volume injectionof an extract, since analytes are already concentrated in the PDMS coating of the stir bar. Stir bar sorptive extraction with the Twister was applied to a 10 mL aliquot of the water sample from theleachables simulation experiment described in the sample preparation section (figure 10). In parallel, a Twisterextraction of a 10 mL blank water sample was performed, and the analytes determined by thermal desorption-GC/MS. The resulting chromatogram of the blank showed a couple of siloxanes from the PDMS coating ofthe Twister stir bar, but no significant traces of other organic compounds. The siloxanes are marked with anasterisk (*) in all following chromatograms. Cyclohexanone 2,6-di-tert-Butyl-p-benzoquinone 2 2-Ethyl hexanol BHT 3 Nonanal 2,4-di-tert-Butyl phenol 4 Nonanol Diethyl phthalate 5 2-tert-Butyl-1,4-benzoquinone 3,5-di-tert-butyl-4-hydroxybenzaldehyde 6 1,3-di-tert-Butyl benzene Isobutyl phthalate 7 Acetyl methylthiophene (?) 7,9-di-tert-butyl-1-oxaspiro-[4.5] 8 Diphenyl ether 12 deca-6,9-diene-2,8-dione 9 1,1-Dimethylethyl-4-methoxyphenol (BHA) 17 DEHP 40.00 45.00 Figure 10. Stir bar sorptive extraction of a 10 mL aliquot of a 250 mL deionized water sample stored for 48hours at 40°C in an IV bag, splitless sample introduction. Figure 11. Stir bar sorptive extraction of a 10 mL aliquot of a 250 mL deionized water sample stored for 48hours at 40C in an IV bag, splitless sample introduction (zoom 15-25 minutes). Figure 12. Stir bar sorptive extraction of a 10 mL aliquot of a 250 mL deionized water sample stored for 48hours at 40°C in an IV bag, splitless sample introduction (zoom 25-33.5 minutes). Using SBSE, a couple ofcompounds could be identified that were also found in the previously mentioned thermalextraction experiments performed on the packaging material. Obviously some of these compounds were leachedinto the aqueous simulant. Additional compounds were detected that had not been detected as extractables,possibly due to their presence at very low concentrations. Some compounds, among them Benzothiazole,N,N-Dibutyl formamide, and a group of compounds that had m/z 125 and 140 as major masses were additionallychecked by means of time-of-flight mass spectrometry. Figure 13 shows the confirmatory TOF-run for Benzothiazole. The presence of N,N-Dibutylformamidecould not be confirmed. +EIEIC(135.0143) Scan 20150331 02 Twister IW bag.D 15.415.515.615.715.8 15.916 16.1 16.216.316.416.516.616.716.816.91717.117.217.317.417.517.617.717.8 17.9 18 18.118.218.3 18.4 18.518.618.718.818.9 19 19.119.2 19.3 19.4 19.5 Counts vs. Acquisition Time (min) Counts vs. Mass-to-Charge (m/z) Figure 13. Stir bar sorptive extraction of a 10 mL aliquot of a 250 mL deionized water sample stored for 48hours at 40℃ in an IV bag, splitless sample introduction. Presence of Benzothiazol confirmed with Time-of-Flight mass spectrometry (mass error: -0.17 mDa=-1.26 ppm). The single quad library results (Wiley 6 and NIST 14) did not show satisfactory results especially for the peakgroup with m/z 125 and 140. Both libraries suggested that these compounds contain sulfur molecules, e.g. Acetylmethylthiophene. Only after the TOF was used for verification could the presence of sulfur in the molecules beexcluded. The findings of the single quad could not be confirmed (figure 14). 16.8 16.9 17 17.117.217.317.417.517.617.7 17.8 17.9 1818.1 18.2 18.3 18.4 18.5 18.6 18.7 18.8 18.919 19.119.2 19.3 19.4 19.5 19.619.719.819.9 20 20.120.2 20.3 20.4 Figure 14. Stir bar sorptive extraction of a 10 mL aliquot of a 250 mL deionized water sample stored for 48hours at 40°℃ in an IV bag, splitless sample introduction. The presence of sulfur-containing compounds withmasses m/z 125 and 140 was refuted through use of Time-of-Flight mass spectrometry. Table 2 lists compounds determined based on stir bar sorptive extraction along with their related source amongthe IV bag components and their possible origin. Table 2. Compounds identified following stir bar sorptive extraction of an aqueous simulant placed in an IVbag listed along with the IV bag component source and possible origin. No. Compound Main Source Possible origin 1 Cyclohexanone IV tubing Residual solvent 2 2-Ethyl hexanol plastic valve DEHP metabolite, intermediate 3 Acetophenon IV bag Residual solvent 4 Nonanal 5 Nonanol 6 2-tert-Butyl-1,4-benzoquinone IV bag BHT metabolite 7 Benzothiazole Vulcanization agent 8 1,3-di-tert-Butyl benzene IV bag Antioxidant degradation product 9 Diphenyl ether Intermediate in the production of surfaceactive agents and high temperature lubricants 10 1,1-Dimethylethyl-4-methoxyphenol (Butylated Hydroxyanisole BHA) IV bag Antioxidant 11 2,6-di(tert-butyl)-4-hydroxy-4-methyl-2,5-cyclohexadien-1-one (BHT-OH) lV bag BHT metabolite 11 2,6-di-tert-Butyl-p-benzoquinone lV bag BHT metabolite 13 2,5-di-tert-Butyl-1,4-benzoquinone(BHT-quinone) lV bag BHT metabolite 14 Butylated Hydroxytoluene BHT plastic valve Antioxidant 15 2,4-di-tert-Butyl phenol lV bag Antioxidant degradation product 16 tert-Butylhydroquinone lV bag Antioxidant 17 Diethyl phthalate plastic valve Plasticizer 18 Benzophenone UV stabilizer 19 2-Ethylhexyl benzoate plastic valve, IV tubing Plasticizer 20 1,4-di-tert-Butylhydroquinone IV bag Antioxidant 21 3,5-di-tert-butyl-4-hydroxybenzaldehyde lV bag BHT metabolite 22 3,5-di-tert-Butyl-4- lV bag BHT metabolite hydroxyacetophenone 23 Isobutyl phthalate IV bag 24 7,9-di-tert-butyl-1-oxaspiro-[4.5]deca-6,9-diene-2,8-dione IV bag degradation product of 2,4-di-tert Butylphenol 25 DEHP plastic valve, IV tubing Plasticizer Thermal Desorption of packaging components followedby Twister analysis of aqueous simulants provides asimple and efficient means of creating a comprehensivetarget list for future leachables experiments. Thermaldesorption, when performed without an in-line valve ortransfer line, can successfully transport heavy SVOC’sto the GCMS with very low carry-over, even betweenseverely overloaded samples, and was shown to be veryuseful in determining the package component sourceof any extractable compound. Also demonstrated was the need for the highresolving power and accurate mass of the GC-QTOF-MS in some cases, both for confirmation and forexclusion of compounds in extractables data (and inleachables data as well). The use ofTwister producedchromatograms rich with trace species, and only afew were discussed here. In general the QTOF is apowerful tool for examining all of these species ifthe sample introduction technique used can introducethem. Coupling simple, rugged, and efficient thermaldesorption sample introduction to the QTOF gives theinstrument an opportunity to rise to its potential. The data presented here was generated fordemonstration of concepts. In order to comply withFDA guidelines, additional replicate measurementsneed to be performed as well as quantitative or semi-quantitative estimates of the extractables. ( “For research use only. Not for use in diagnostic procedures."The information provided for this product is intended for referenceand research purposes only. GERSTEL offers no guarantee a s tothe quality and suitability of this data for your specific application.Information, descriptions a n d specifications in this publicationare subject to change without notice. ) ( REFERENCES ) ( [1 ] F DA. G u idance for industry: c o ntainer-closuresystems for packaging human drugs and biologics.Rockville, MD: FDA; 1999. ) ( 2] USP1664.Assessment ofDrug Product LeachablesAssociated with Pharmaceutical PackagingDelivery Systems. Rockville, MD; 2013. ) ( 3]Method Development and Validation for t heDetermination o f 2,4,6-Tribromoanisole,2,4,6-Tribromophenol, 2,4,6-Trichloroanisole,and 2,4,6-Trichlorophenol in Various DrugProducts u sing Stir Bar Sorptive Extraction andGas Chromatography-Tandem M a ss SpectrometryDetection, Jiun-Tang Huang, Lori Alquier, JoyceP. Kaisa,Gai l Reed,Timothy Gilmor, Gyorgy Vas,Journal of Chromatography A (2012). ) ( [4 ] Stir bar sorptive extraction combined with GC-MS/MS for determination oflow level leachablecomponents from implantable medical de v ices.Barbara L Armstrong, Askim F Senyurt, VenkatNarayan, Xiande Wang, Lori A lquier, GyorgyVas, Journal of Pharmaceutical and BiomedicalAnalysis 02/2013; 7 4:162-70. ) ( [5 ] Use ofThermal Desorption GC-MS to CharacterizePackaging Materials for Potential Extractables,Cindy Z weiben, Arthur J. S haw, Journal o fPharmaceutical Science and Technology Vol. 63, N o.4, July-August 2009 ) ( [6 ] ] Cyclohexanone contamination fromextracorporeal circuits i mpairs cardiovascularfunction, Thompson-Torgerson CS, ChampionHC, S anthanam L, Harris ZL, S houkas AA,Am J Physiol H eart Circ P hysiol. 2009Jun;296(6):H1926-32. ) [7] Stir Bar Sorptive Extraction (SBSE),a NovelExtraction Technique for Aqueous Samples:Theory and Principle, Erik Baltussen, PatSandra, Frank David, Carel Cramers, Journal ofMicrocolumn Separations 11: 737-747,1999 GERSTEL GmbH & Co. KG Eberhard-Gerstel-Platz 1 45473 Mulheim an der RuhrGermany+49 (0)208-765 03-0+49 (0) 208-765 03 33 gerstel@gerstel.com www.gerstel.com GERSTEL Worldwide GERSTEL LLP GERSTEL, Inc. GERSTELAG GERSTEL K.K. 701 Digital Drive, Suite J Wassergrabe 27 1-3-1 Nakane,Meguro-ku Linthicum, MD 21090 CH-6210 Sursee Tokyo 152-0031 USA Switzerland SMBC Toritsudai Ekimae Bldg 4F +1 (410) 247 5885 +41 (41) 9 21 97 23 Japan +1 (410) 247 5887 +41 (41)921 97 25 +81357315321 sales@gerstelus.com swiss@ch.gerstel.com +81 357315322 www.gerstelus.com www.gerstel.ch info@gerstel.co.jp Information, descriptions and specifications in thisPublication are subject to change without notice.GERSTEL, GRAPHPACK and TWISTER are registeredtrademarks of GERSTEL GmbH & Co. KG. www.gerstel.co.jp GERSTEL (Shanghai) Co. Ltd GERSTEL Brasil 10 Science Park Road Room 206,2F,Bldg.56 Av. Pascoal da Rocha Falcao, 367 #02-18 The Alpha No.1000,Jinhai Road, 04785-000 Sao Paulo -SP Brasil Singapore 117684 Pudong District +55(11)5665-8931 +65 6779 0933 Shanghai 201206 +55 (11)5666-9084 +65 6779 0938 +86 21 50 93 30 57 gerstel-brasil@gerstel.com SEA@gerstel.com china@gerstel.com www.gerstel.com.br www.gerstel.com # www.gerstel.cn AN/ 介绍:用于医药产品的包装可能含有一些杂质,其可能与产品接触,从而导致产品的变化。为了评估包装系统,可以在包装里填充一个接近药品的化学成分的仿真物。使用较高的温度和/或更具攻击性的模拟条件可用于加速这些化合物的提取和转移到仿真物中。在这些条件下从包装中引入仿真物的化合物称为可萃取物。如果药品本身在正常条件下储存后进行分析,则检测到的来自包装化合物称为可溶出物。因为这两项测试通常都是在完整的包装系统上进行的,所以具体了解包装上哪一部分造是罪魁祸首就需要额外的化学鉴定工作。静脉输液袋中的溶液直接大量流入患者的静脉,因此对含有水性介质的静脉输液袋的可萃取物和可溶出物的研究尤为重要。 这些袋子还可以在医院药房中装满各种药品,从而使传统的可溶出物实验(仅对一种药品进行测试)的相关性降低,并且难以执行。 因此,可萃取物实验的结果甚至更为重要。实验步骤:1.高温下对输液袋组件的直接热脱附(萃取)2.对微温下储存在袋子中的水仿真物进行搅拌吸附萃取(SBSE-GC/MS)3. 在需要时使用SBSE-GC-QTOF-MS来解决步骤2中无法解决的物质鉴定问题步骤1非常重要:在高温(200°C)下直接解析单个输液袋的组分,可以为步骤2中检测出的溶出物分析提供可萃取物候选者的“名单”。更重要的是,对每个输液袋组分进行直接热脱附检测,可以更容易将检测到的化合物关联到相对应的输液袋组分中可溶出物的鉴定中。结果与讨论:对医用输液袋进行直接热萃取分析在高温下对固体材料的热萃取,可以用来分析挥发性和半挥发性物质,而与它们的极性无关。这项技术的易用性和有效性使得可以对最相关的化合物,即具有相对高迁移率的化合物,进行快速而大量的可萃取性研究。这是热萃取技术相对于溶剂萃取技术在对可萃取化合物的分析上的主要优势,因为在溶剂萃取中,分析物的萃取率更多地取决于溶剂的极性,而不是它们的挥发性。并且热萃取法可最大限度地减少样品制备,并消除溶剂对样品的污染。对应的化合物名称:1.环己酮, 2. 2-乙基己醇, 3. 1,3-二叔丁基苯, 4, 二苯醚, 5. 2,6-二叔丁基-对-苯醌, 6. 丁基化羟基甲苯 (BHT), 7.2,4-二叔丁基苯酚, 8. 邻苯二甲酸二乙酯 (DEP), 9. 苯乙酸2-乙基己酯, 10. 水杨酸2-乙基己酯, 11. 邻苯二甲酸二乙酯 (DEHP)下图显示了使用完全相同的仪器条件在分析后立即使用空管得到的空白色谱图和之前样品超载的色谱图的叠加图。可以看出,几乎没有样品残留。因此,即使是高浓度SVOC的样品也可以进行分析,而不会影响后续分析的准确性。对水性仿真物的搅拌棒吸附萃取(SBSE)搅拌棒吸附萃取(SBSE)是一种无溶剂的样品萃取技术,用于从水性样品中富集化合物,最早由Baltussen等人在1999年引入。 SBSE可以最大程度地减少样品制备,并避免了从水性样品中提取分析物通常需要的有机溶剂对样品的污染。另外,由于分析物已经在搅拌棒的PDMS涂层中浓缩,因此不需要对萃取液进行大体积注射从而实现进一步的浓缩过程。将Twister搅拌棒吸附萃取法应用于样品制备部分中所述的可溶出物仿真实验的10 mL水样中(下图)。同时,用Twister再平行萃取10 mL空白水样品,并通过热脱附GC / MS测定分析物。SBSE搅拌棒吸附萃取250 mL去离子水样品的10 mL等分试样,该样品在输液袋中在40°C下储存了48小时,不分流进样。1. 环己酮, 2. 2-乙基己醇, 3. 壬醛, 4. 壬酮, 5. 2-叔丁基-1,4-苯醌, 6. 1,3-二叔丁基苯, 7. 乙酰甲基噻吩(?), 8. 二苯醚, 9. 1,1-二甲基-4-甲氧基苯酚(BHA), 10. 2,6-二叔丁基对苯醌,11. 2,6-二叔丁基羟基甲苯(BHT), 12. 2,4-二叔丁基苯酚, 13. 邻苯二甲酸二乙酯, 14.3,5-二叔丁基 - 羟基苯甲醛 15.邻苯二甲酸异丁酯 16. 7,9-二 - 叔丁基-1-氧杂螺-4,5-癸-6,9-二烯-2,8-二酮 17. DEHP 邻苯二甲酸二(2-乙基己)酯结论对输液袋的热解吸和使用SBSE萃取仿真水样品的分析是一种简单有效的样品分析方法,可以为将来的溶出实验创建一个全面的目标物列表。在没有串联阀或传输线的情况下进行热解析,可以成功地将高沸点化合物SVOC输送到GCMS,并且保证低残留,即使在样品严重超载时,也没有问题。此方法对确定任何包采种的可萃取化合物的来源都非常有用。还证明了在某些情况下,GC-QTOFMS需要高分辨率和准确质量,以确认和排除可提取数据(以及可提取数据)中的化合物。利用Twister得到的色谱图中含有丰富的微量化合物,本文仅对其中的一些进行了讨论。一般来说,QTOF是检验所有这些物种的有力工具,只要所使用的前处理技术能够导入化合物即可。将简单、坚固、高效的热脱附样品引入QTOF,使仪器有机会发挥其潜力。
关闭-
1/16

-
2/16
还剩14页未读,是否继续阅读?
继续免费阅读全文产品配置单
GERSTEL(哲斯泰)为您提供《材料,医用输液袋中可萃取物,可溶出物检测方案(其它萃取设备)》,该方案主要用于包装中可萃取物,可溶出物检测,参考标准《暂无》,《材料,医用输液袋中可萃取物,可溶出物检测方案(其它萃取设备)》用到的仪器有GERSTEL 搅拌棒Twister (萃取、固相微萃取)、GERSTEL热脱附单元TDU2 (热解吸,热解析)。
我要纠错
推荐专场
固相微萃取仪、固相微萃取装置
更多相关方案







 咨询
咨询



