方案详情文
智能文字提取功能测试中
International Journal of Pharmaceutics 575 (2020) 118875 Contents lists available at ScienceDirect International Journal of Pharmaceutics ELSEVIER journal homepage: www.elsevier.com/locate/ijpharm Taste-masking and colloidal-stable cubosomes loaded with Cefpodoximeproxetil for pediatric oral delivery Yanliang Fan, Hsinyi Chen, Zhengwei Huang, Jianzheng Zhu , Faiiam Wan, Tingting Peng,Xin Pan,Ying Huang , Chuanbin Wu,** “School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou 510006, Guangdong. PR China'College of Pharmacy, Jinan University, Guangzhou 510632, Guangdong, PR China ARTICLEINFO ABSTR A C T Keywords:Lyotropic liquid crystalTaste-maskingCefpodoxime proxetilPediatric oral delivery Drug administration failure has been often witnessed in pediatric due to children’s resistance to take medicineswith bitter taste. Taste-masking is the key requirement among the scanty drugs available for children. Solid taste-masking systems, such as tablets and capsules, are difficult to swallow for children. Therefore, a liquid taste-masking system based on lyotropic liquid crystalline nanoparticles (LLCNs) was developed in this study.Cefpodoxime proxetil (CFP), a typically bitter drug used as antibiotic in pediatric, was selected as the modeldrug, and the encapsulation of CFP into the LLCNs was envisaged to improve their taste. Pluronic F127 wasadded to improve the colloidal stability of CFP-LLCNs. The optimized CFP-LLCNs showed the particle size of187.29 ± 4.12 nm and the encapsulation efficiency of 85.80%. The mesophase analysis by polarized lightmicroscopy and small angle X-ray scattering confirmed the cubic phase of CFP-LLCNs. It showed a sustained-release profile well fitted to Higuchi model, indicating that diffusion and erosion were both responsible for theCFP release. The taste-masking ability of CFP-LLCNs was confirmed by electronic tongue, compared to CFP andcommercial product. The colloidal stability was verified after 3 months storage in room condition (25 ± 2℃,70 ±2%RH). To sum up, the taste-masking and colloidal-stable CFP-LLCNs showed great potential for pediatricoral delivery. 1. Introduction According to the Capital Institute of Pediatrics and SFDA (HUATAISECURITIES CO., 2015), pediatric patients account for about 20% ofthe total patient population in China, while 90% of clinical medicine isnot available for pediatric patients. To be precise, only 1.55% drugs arespecifically designed for pediatric patients. The metabolism, excretionand tolerance of many drugs for children are poor, due to their im-perfect development of liver, kidney, central nervous system and en-docrine system (Huang Beiru, 2019). Among the scanty drugs availableto children, poor taste has become a big problem. Drug administrationfailure has been often witnessed in pediatric due to children’s resistanceto medicines with bitter taste. In order to reduce the resistance of pediatric patients to bitter taste,taste masking techniques are commonly used to increase the com-pliance of pediatric patients. Basically, there are two types of taste-masking drug delivery systems: solid taste-masking systems and liquid taste-masking systems. Solid taste-masking system mainly employedcapsulation and coating techniques to hinder drug from directly con-I-tacting with taste buds. However, it is hard for children to swallowcapsules and tablets. Moreover, it is inconvenient to adjust the dose forchildren at different ages due to their fixed dose (Lam et al., 2014).Therefore, liquid taste-masking system is preferential for pediatric oraldelivery. The bitterness of drug is sensitized when the drug dissolves in salivaand comes in contact with taste buds (Al-Kasmi et al.,2017). In view ofthis, there are three ways to mask the taste: (1) adding taste correctivesto confuse the taste buds or reversible and temporarily paralyze tastebuds, such as oral liquid using sweeteners and peppermint oil, which isineffective for drugs with strong bitter taste; (2) competing with drugsfor bitter receptors or blocking bitter signals sent to the brain, which islimited in lipoproteins and phospholipids masking the bitterness ofquinine and propranolol (Katsuragi and Kurihara, 1993; Katsuragiet al, 1996); (3) encapsulating drugs with carriers to avoid their ( E -mail addresses : fan yli an g@ ma i l 2 . s y s u.edu.cn ( Y . Fan) , h zhen g w 3@m ai l 2 .s y s u .e d u . c n ( Z. Huang), p a n xin 2 @m a i l . sy s u . e du.c n (X . P a n), ) ( h u ang y2007@ 1 6 3 .com (Y . Huang), w u c h uan b @ma il . sy s u . e du. c n ( C . Wu). ) ( h t t p s: // d o i. o r g / 1 0 .1016/ j . i jph a r m.2 01 9 . 1 1 88 75 ) ( Received 1 6 September 2019; Received in r evised form 6 November 2019; A c cepted 1 2 N o vember 2019 ) ( Available online 22 November 2019 ) contact with taste buds, such as microcapsules and cyclodextrin (Boraet al., 2008; Liu et al., 2019). For the third approach, toxic organicsolvent was ineluctable in the preparation of microcapsules, and drugswhich can be encapsulated in cyclodextrin were limited by their mo-lecule polarity and weight. Thus, it is urgent to develop a novel andreliable taste-masking drug delivery system for pediatric oral delivery. Lyotropic liquid crystalline nanoparticles (LLCNs) are expected tobe a promising candidate. LLCNs have large internal and external sur-face area, which is theoretically ideal for drug encapsulation (Demurtaset al., 2015; Rajabalaya et al., 2017). Besides, LLCNs own a highly or-dered bicontinuous cubic or honeycombed nanostructure of waterchannels through the lipid matrix (Azhari et al., 2016) (Fig. Sli).Consequently, drug can be encapsulated in the water channels or lipidbilayers of LLCNs, leading to isolation from taste buds (Fig. Slii),making it possible for taste-masking. Moreover, the oral liquid form ofLLCNs is easier to swallow and adjust the dosage in pediatric. However, LLCNs are not colloidally stable and the particle size isdifficult to control. Colloidal instability and uncontrollable particle sizemay lead to the leakage of encapsulated drugs, resulting in the failure oftaste-masking. In this work, stabilizer Pluronic F127 was added to avoidparticle aggregation and high-pressure homogenization (HPH) was usedfor preparation to get a controllable particle size (Akbar et al., 2017;Akhlaghi et al., 2016). Another issue for developing anovel and reliable liquid taste-masking system based on LLCNs is to establish a reliable analyticalmethod of tastes. Evaluating the taste by human volunteers was acommon method because of directness and clinical necessity (Schiffmanet al., 2000). However, to a certain sample, different volunteers haddifferent subjective views on it, which is called the individual differ-ences. A large sample size was required, otherwise the deviation ofresults could be too large to work as reference for clinical use. There-fore, it is not suitable for the small-scale laboratory experiments.Electronic tongue provides fast objective rating of different tastes withlow cost, and it is free of ethics application and enrollment of volun-teers. Thus,electronic tongue is well fitted to the lab scale and moresuitable in the preclinical research. In this study, electronic tongue wasused to analyze, recognize and judge the taste of tested samples by si-mulating human's tongue (Di Rosa et al., 2017; Woertz et al., 2011). Itconsists of taste sensor array, signal acquisition system and patternrecognition system. It can evaluate five basic tastes (sourness, sweet-ness, bitterness, saltiness and umami) and astringency by detecting thechanges in membrane potential caused by electrostatic interaction orhydrophobic interaction between various taste substances and the ar-tificial lipid membrane (Woertz et al., 2011) (Fig. 1). Compared withtaste by volunteers, electronic tongue is more objective, safe and con-venient. Cefpodoxime proxetil (CFP) (Fig.S2) was selected as the model drugwith bitter taste. CFP is a third-generation cephalosporin antibiotic anda pro-drug for oral administration, which is characterized by its broad-spectrum activity against gram-positive and gram-negative micro-organisms (MarkTodd, 1994; Mujtaba and Kohli, 2016). CFP is fre-quently used in pediatric oral delivery for its safety and efficacy(Manhua, 2016; San Joaquin and Stull, 2000, Zhou Hong et al., 2012).The marketed CFP products, including tablet, capsule and dry suspen-sion (Commission, 2010), have low compliance for pediatric patientsdue to the difficulty to swallow or unpalatable taste. Therefore, LLCNswere applied to encapsulate CFP and the taste-masking efficiency ofLLCNs was also evaluated. The aim of this study was to develop a colloid-stable CFP-loadedlyotropic liquid crystalline nanoparticles system (CFP-LLCNs) withgood taste-masking property. The prepared LLCNs system will helpincrease the compliance of the oral delivery for pediatric patients. Ofnote, the taste-masking property of LLCNs has not been reported, and itis interesting to verify our assumption about the taste-masking propertyof LLCNs. 2. Materials and methods 2.1. Materials Cefpodoximeproxetilil(CFP)wasobtained from ZhixingBiotechnology Co., Ltd. (Guangzhou, China). Phytantriol (PYT) waspurchased from DSM (Venlo, Nederland). Pluronic F127 (F127) waspurchased from BASF SE (Lundwigshafen, Germany). 1,2-propandiol(propylene glycol, purity >99.0%) and ethanol were purchased fromZhiyuan Co., Ltd. (Tianjin, China). Methanol (purity≥99.9%) waspurchased from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin,China). Coconut flavor and peppermint were purchased from RaynerLimited (Worthing, UK). Cefpodoxime Proxetil For Suspension (SFDAapproval number H20040210) was purchased from Hainan SanyePharmaceutical Factory Co., Ltd (Hainan, China). 2.2. Preparation of CFP-LLCNs CFP-LLCNs were prepared by a top-down approach (Akbar et al.,2017). PYT mixed with propylene glycol (PG) and Pluronic F127 wereheated at 50-60 ℃ in water bath until complete melting, and then CFPwas added to the mixture for dissolving to get CFP loaded precursor.After that, precursor was gradually added to the ultrapure water, withan Ultra-Turrax high shear mixer (FA25 model,FLUKO, Shanghai) at1,000 rpm for 10 min. Dispersions were thereafter homogenized using ahigh-pressure homogenizer (ATS Engineering Co., Ltd, Jiangsu) to form Fig. 1. The mechanism of electronic tongue: artificial lipid membrane sensing technology similar to the working principle of taste bud cells. (A) When there are tastecompounds on the surface of biomembrane, membrane potential changes will be generated. This information will be transmitted to the brain through the neuralnetwork and become our cognition “taste". (B) The change of membrane potential is produced by the interaction between the artificial lipid membrane and the tastecompounds through electrostatic or hydrophobic interaction. The potential is used as the output signal of the sensor to transmit to the computer for analysis, so as torecognize the taste intensity and taste characteristics. Table 1Information of tested samples. Number Sample API Concentration Name Note KCl (aq) 0 KCl Background 2 Blank LLCNs 0 B Positive Control 3 CFP-LLCNs 0.15 g/90 mL F 4 Vanila CFP-LLCNs 0.15 g/90 mL Fv 5 Peppermint CFP- 0.15 g/90 mL Fp LLCNs 6 Coconut CFP-LLCNs 0.15 g/90 mL Fc 7 Commodity 0.15 g/90 mL Commercial Control Negative Control 8 API 0.15 g/90 mL A an opalescent dispersion of the cubic nanoparticles. The samples werestored for 24 h at room temperature to equilibrate prior to analysis. In order to achieve CFP-LLCNs (containing 50 mg CFP) with ap-propriate particle size and high encapsulation efficiency, single factorexperiment was carried out. Parameters of high-pressure homogenizerincluded 2 factors (pressure and cycle) and 3 levels (800 bar, 1000 barand 1200 bar; 5 cycles, 10 cycles and 20 cycles). Particle size and PDIwere used to evaluate different parameters of high-pressure homo-genizer and mass ratios (4:1,9:1) of PYT to F127 (Akhlaghi et al.,2016;Dian et al., 2013). Similarly, encapsulation efficiency (EE) was used tooptimize the contents of PYT (1.0 g, 1.5 g and 2.0 g) and PG (1.0 g,1.5 gand 2.0 g). The details can be found in the supplementary materialTab. S2. 2.3. Characterizations of CFP-LLCNs 2.3.1. Particle size and polydispersity index (PDI) Particle size and PDI of CFP-LLCNs were measured by ZetasizerNano ZS90 (Malvern Instruments, Malvern, UK). The samples werediluted with ultrapure water before measurement. Particle size wasanalyzed by the Dispersion Technology Software provided by MalvernInstruments. 2.3.2. Encapsulation efficiency (EE) The CFP content in the LLCNs was determined by ultrafiltrationcentrifugation method. The CFP-LLCNs (1 mL) were placed in a 15 mLultrafiltration centrifugal tube (30KD, Millipore). The dispersions werethen centrifuged at 4000 rpm for 30 min. The subnatant was assayedspectrophotometrically (max =238 nm; TU-1901, Persee, Beijing) forCFP content. The measurements were performed in triplicate and EEwas calculated according to the following equation: 2.3.3. Polarized light microscopy (PLM) Samples were inspected under a light microscope (ECLIPSE LV100NPOL, Nikon, Japan) fitted with polarizing filters at ambient tempera-ture. Images were captured using a digital camera (Y-TV55, Nikon,Japan) and classified according to the visualized textures of lyotropic Table 2 mesophases. 2.3.4. Small angle X-ray scattering (SAXS) For phase structural determination of CFP-LLCNs, SAXS measure-ments were performed (Boge et al., 2017) at SAXSess camera (Anton-Paar, Graz, Austria). A PW3830 X-ray generator with a long fine focussealed glass X-ray tube (PANalytical) was operated at 40 kV and 50 mA.Samples were positioned in a capillary of 1 mm diameter and 0.01 mmwall thickness. The sample-to-detector distance was 261.2 mm, and thetemperature was kept at 26.0C. The 2D scattered intensity distributionrecorded by an imaging-plate (IP) detector was read out by a Cyclonestorage phosphor system (Perkin Elmer, USA). The 2D data were in-tegrated into the one-dimensional scattering function I(q) as a functionof the magnitude of the scattering vector q (q= 4nsin0/A, where 20 isthe scattering angle). Each measurement was collected for 30 min. AllI(q) data were normalized so as to have the uniform primary intensity atq = 0 for transmission calibration. The background scattering con-tributions from capillary and solvent were corrected. 2.4. In vitro release studies The release profiles of CFP from LLCNs were examined in simulatedgastric fluid (SGF, pH 1.2) and simulated intestinal fluid (SIF, pH6.8)by the dialysis method. The CFP-LLCNs (3 mL) was transferred into adialysis bag. The release of CFP from LLCNs (equivalent to 5 mg ofdrug) was investigated in 50 mL dissolution medium, and incubated at37 C with an agitation speed of 100 rpm using the Thermostatsteambath vibrator (THZ-82C, Baita Jinchang Experimental InstrumentFactory, Jiangsu). Samples were initially released in SGF for 2 h. After2 h, the pH of dissolution medium was changed from 1.2 to 6.8 for thenext 22 h. At predetermined time intervals (0 h, 0.5 h, 1.0 h, 2.0 h,3.0 h, 4.0 h, 5.0 h,6.0 h, 8.0 h, 12.0 h and 24 h), all the dissolutionmedium was withdrawn and replaced with fresh medium. These sam-ples were diluted, and the drug content was determined spectro-photometrically at 264 nm using ultraviolet photometer. The controlgroup (water suspension of 5 mg CFP) was taken as a reference. Eachexperiment was repeated three times. 2.5. Release kinetics study Data obtained from in vitro drug release studies were plotted invarious kinetic models: Zero order model: First order model: Higuchi model: Fig. 2. Measurement method of taste sensors. In order to further explore dissolution mechanisms of LLCNs, therelease data were fitted with following formula: Korsmeyer-Peppas model: where M and M.. represent the accumulated drug release at tand(total drug content) time, respectively. k is the kinetic constant and ndescribes the kinetic index. To evaluate the drug release with changes in the surface area andthe diameter of the particles, the data were also plotted using theHixson-Crowell cube root law: where . is the amount of drug released in time t, Qo is the initialamount of the drug in the nanoparticles, and KHc is the rate constant forthe Hixson-Crowell rate equation, as the cube root of the percentage ofdrug remaining in the matrix vs. time. 2.6. Electronic tongue Electronic tongue (TS-5000Z, INSENT, Japan) was used to test CFP-LLCNs and CFP-LLCNs with different essence. Blank LLCNs was set aspositive control and CFP suspension (API) was set as negative control.Cefpodoxime Proxetil For Suspension (Commodity) was used as acommercial control. The information of every group was showed inTable 1 and the preparation could be found in supplementary material.KCl solution (90 mL, 10 mM) was used as background. The tastes of samples were evaluated separately 3 times by 4 sensorsand 6 parameters. The information of sensors is in Table 2 and opera-tion process is in Fig. 2. Using the potential ofa reference solution aszero, the difference in potential with the samplesolution is measured asthe initial taste. The sensors are then lightly washed and the differencein potential with the reference solution is measured as the aftertaste.Principal component analysis (PCA) was used to analyze these data byINSENT Software (Lenik et al., 2016; Rachid et al., 2010). 2.7.1. Conditional stability High temperature test: CFP-LLCNs were placed in thermostat (DHG-9140A, Keelrein, Shanghai) at 60℃ (n=3). The samples were col-lected at different time intervals (0 d,5 d, 10 d and 30 d) and thencharacterized by particle size, PDI and encapsulation efficiency. Strong illumination test: CFP-loaded LLCNs were placed in light box(YP-SD150T, SUYING) at 5275 lx, 25 ±0.2 ℃, 47±0.3%RH(n=3). The samples were collected at different time intervals (0 d,5 d,10 d and 30 d) and then subjected to measurement of particle size, PDIand encapsulation efficiency. 2.7.2. Stability evaluation CFP-LLCNs were placed in room condition (25 ± 2℃, 70 ± 2%RH) for 3 months (n = 3). The particle size, PDI and encapsulationefficiency of samples were determined once a week in the first month.After 1 month, the samples were tested once a month. 2.8. Statistical analysis Results are shown as mean ± SD from at least three measurements.Statistical difference between the mean values was calculated usingANOVA one-way analysis (Origin 2017, OriginLab Corporation, US).Probability values p< 0.05 were considered significant. 3. Results and discussion Glyceryl monoglycerides (GMO) and phytantriol (PYT) (Fig. S2)A.were the most commonly used materials to generate the cubic meso-phases in LLCNs for drug delivery. PYT has gained more interest in thebiomedical industry because of its superior chemical stability andhigher purity (Parinaz Akhlaghi and Loh, 2017). Hence, PYT basedLLCNs were established in this study. 摘要:由于儿童对苦味药物的耐药,儿科经常出现用药失败的情况。味觉掩蔽是少有的儿童药物中的关键要求。固体味觉掩蔽系统,如片剂和胶囊,是很难下咽的儿童。因此,本研究开发了一种基于溶致性液晶纳米粒子(LLCNs)的液体味觉掩蔽系统(LLCNs),选择儿童常用的苦味药物头孢泊肟丙酯(CFP)作为模型药物,并设想将CFP包封于LLCNs中以提高其口感。为提高CFP-LLCNs的胶体稳定性,加入Pluronic F 127优化后的CFP-LLCNS粒径为187.29±4.12nm,包封率为85.80%。通过偏光显微镜和小角X射线散射的中间相分析,证实了CFP-LLCNs的立方相。其缓释剖面符合Higuchi模型,表明扩散和侵蚀对CFP的释放都有影响。通过电子舌验证了CFP-LLCNs的味觉掩蔽能力,并与CFP和商品化产品进行了比较.在室温(25±2度,70±2%RH)条件下保存3个月后,胶体稳定性得到验证。总之,味觉掩蔽和胶体稳定的CFP-LLCNs在儿科中显示出巨大的潜力。
关闭-
1/10

-
2/10

还剩8页未读,是否继续阅读?
继续免费阅读全文产品配置单
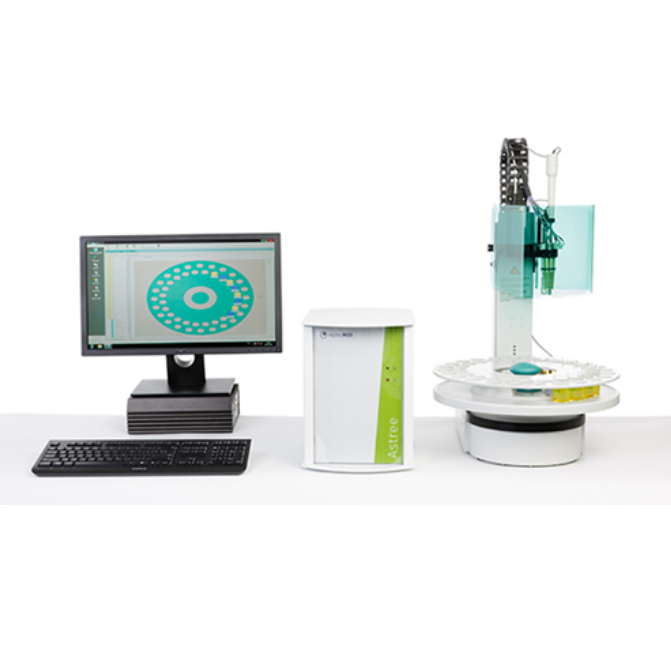
北京盈盛恒泰科技有限责任公司为您提供《小儿口服药中苦味掩蔽效果检测方案(感官智能分析)》,该方案主要用于化药制剂中其他检测,参考标准《暂无》,《小儿口服药中苦味掩蔽效果检测方案(感官智能分析)》用到的仪器有日本INSENT味觉分析系统(电子舌)、电子舌。
我要纠错
推荐专场
感官智能分析系统(电子鼻/电子舌)
更多相关方案





 咨询
咨询





