厨房洗涤剂中阴离子表面活性剂含量检测方案(自动电位滴定)
检测样品 洗涤剂
检测项目 含量分析
方案详情文
智能文字提取功能测试中
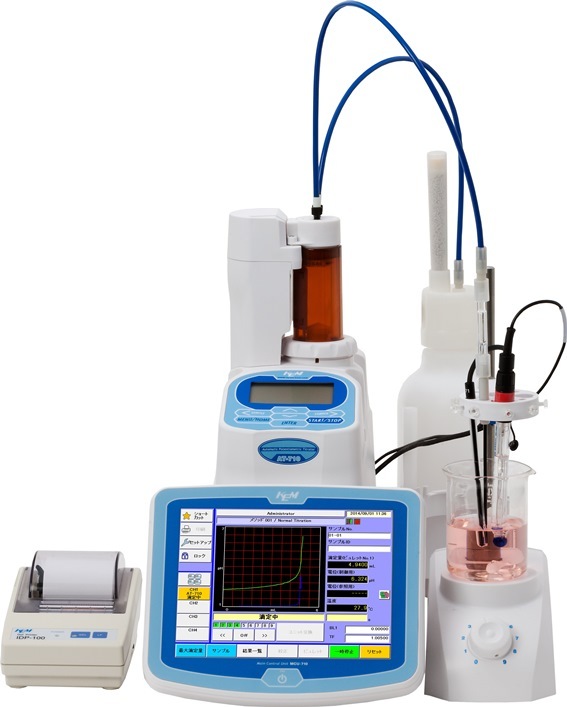
阴离子表面活性剂含量的测定 应用资料表面活性剂分为阳离子表面活性剂、阴离子表面活性剂、两性表面活性剂和非离子表面活性剂。本应用资料使用两种检测方式(表面活性剂电极和粒子电荷检测器)测量厨房洗涤剂中所含阴离子表面活性剂的含量。京都電子工業株式会社KYOTO ELECTRONICSMANUFACTURING CO.,LTD.APAT-0149er APAT-0149er Application Note Measurement of anionic surfactants in kitchen detergent byusing surfactant electrode and particle charge detector Industry Cosmetics & soapInstrument Automatic potentiometric titratorMeasurement method Potentiometric titration / Ion-association titrationStandards QB/T 4970 1. Scope Surfactant is a compound having hydrophobic and hydrophilic parts in the molecule. Dependingon the properties of the hydrophilic part, surfactant is classified into cationic surfactant, anionicsurfactant, amphoteric surfactant, and nonionic surfactant. In these surfactants, cationic surfactants have a property which the hydrophilic group has apositive electric charge after ionic dissociation, whereas anionic surfactants have a negativecharge. Both of these electric charges react and produce association formation. The producedassociation formation is insoluble and precipitated in water by decreasing ofelectric charge andincreasing of hydrophobicity during the reaction. Potentiometric titration method uses thischemical reaction to directly titrate the ionic surfactant. This Application Note introduces thecase which the anionic surfactants contain in kitchen detergent is measured by using two kindsof detectors (surfactant electrode and particle charge detector). 2. Precautions The sensing part of the surfactant electrode does not have a high resistance to organic solvents.Therefore this part should not be cleaned with organic solvents. After measurement, theelectrode should be rinsed with distilled water and wiped off water with paper towel from thesensing part of electrode. When the particle charge detector is used, immerse the probe in ethanol after eachmeasurement and clean by driving the piston for approximately 1 minute. After that,completely remove the ethanol by rinsing it out with distilled water before starting the nextmeasurement. 3. Post-measurement procedure When the surfactant electrode is used, remove the internal solution and rinse the inside withdistilled water several times by using dropper. After the water from inside, attach the suppliedprotective cap and keep it dry. In the case that the electrode is stored with distilled waterdeterioration of the electrode is accelerated due to elution of the ingredients elements of fromthe sensitive membrane into the solution. When the particle charge detector is used, immerse the probe into ethanol, and rinse by drivingthe piston for approximately 1 minute. Remove the ethanol by rinsing with distilled water, andwipe off away water with a paper towel from the probe and keep the probe dry. 4. Apparatus ·Automatic potentiometric titrator (preamplifier : STD) ·Combined surfactant electrode (Internal liquid: 3.3mol / L potassium chloride solution) ·Particle charge detector 京都电子工业株式会社-可睦电子(上海)商贸有限公司 上海市徐汇区宜山路333号1201室 电话:021-54488867 电邮: kemu-kem@163.com 网址: http://www.kem-china.com 5.Reagents Titrant:0.004mol/L benzethonium chloride Solvent : Distilled water 6. Procedure 1) Take 2.5 g kitchen detergent precisely into a beaker. 2) Add distilled water until 500mL. 3) Take 5mL the sample solution precisely in another beaker, and add 100mL distilled water. 4) Titrate sample solution with 0.004 mol/L of benzethonium chloride solution, and detectinflection point as end-point in titration curve. 7.Calculation Measurement result by using surfactant electrode (%)=EP1xTFxC1xK1/(S/R)Measurement result by using particle charge detector (%)=EP2xTFxC1xK1/(S/R) EP1 ... Titration volume of 1st end point(mL) EP2 ·.Titration volume of 2nd end point (mL) ···Factor of titrant =0.9819*CKSR ...Coefficient of concentration conversion =1.496mg/mL ·..Coefficient of unit conversion =0.1 ..Sam···ple size (g) ·.Purity, dilution factor =100 *The factor was calculated from the mass and the purity of benzethonium chloride solution whenthe titrant is prepared. 8. Example -Titration parameter- *Below parameter was used when measurement by using a surfactant electrode wasperformed.
-
1/4

-
2/4

还剩2页未读,是否继续阅读?
继续免费阅读全文产品配置单
可睦电子(上海)商贸有限公司-日本京都电子(KEM)为您提供《厨房洗涤剂中阴离子表面活性剂含量检测方案(自动电位滴定)》,该方案主要用于洗涤剂中含量分析检测,参考标准《暂无》,《厨房洗涤剂中阴离子表面活性剂含量检测方案(自动电位滴定)》用到的仪器有AT-710S豪华型自动电位滴定仪、AT-710M四通道旗舰型自动电位滴定仪。
我要纠错
推荐专场
相关方案






 咨询
咨询
